How Is the Orbital Diagram of Indium Defined?
The orbital diagram of indium is a visual representation showing how electrons occupy atomic orbitals in an indium atom. Instead of listing electrons numerically, the indium orbital diagram uses boxes and arrows to clearly display orbital occupancy and electron spin, making it ideal for diagram-based explanations.
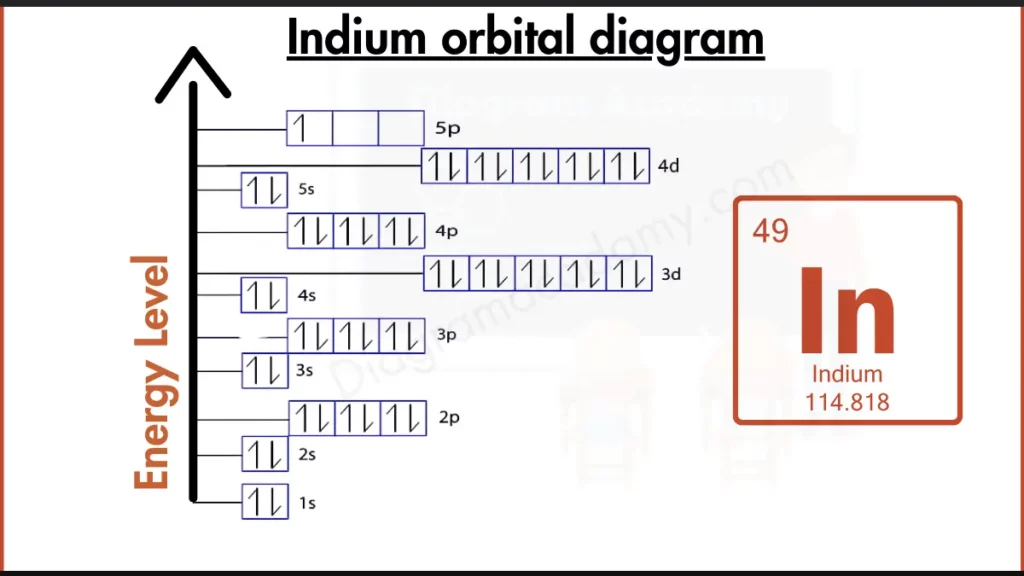
Which Atomic Properties Affect the Indium Orbital Diagram?
Indium has the chemical symbol In and an atomic number of 49, meaning it contains 49 electrons. It belongs to period 5 and the p-block of the periodic table. These properties directly influence the structure of the orbital diagram of indium, especially the involvement of d and p orbitals.
How Does Electron Configuration Translate into the Orbital Diagram of Indium?
The electron configuration of indium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p¹
This configuration is converted into the orbital diagram of indium by assigning each orbital a box and placing electrons as arrows according to filling rules.
How Are Electrons Arranged in the Indium Orbital Filling Diagram?
In the orbital diagram of indium, electrons fill orbitals from lower to higher energy following the Aufbau principle. The 5s orbital fills before the 4d orbitals, and finally one electron enters the 5p orbital. This single p electron is clearly visible in the diagram.
How Can Energy Levels Be Read in an Indium Orbital Diagram?
The indium orbital diagram is arranged vertically to show increasing energy levels. Lower orbitals appear at the bottom, while higher-energy orbitals such as 5p appear at the top. This layout helps readers visually interpret electron energy distribution.
How Is Orbital Notation for Indium Represented?
Orbital notation for indium uses arrows to represent electron spin. All filled orbitals contain paired electrons, while the 5p orbital shows one unpaired electron, explaining indium’s valence behavior.
Why Is the Orbital Diagram of Indium Useful for Learning Chemistry?
The orbital diagram of indium helps explain bonding, oxidation states, and periodic trends. It is especially useful in textbooks, exams, and image-based learning platforms where visual clarity is essential.
Frequently Asked FAQs.
Here are answer about Orbital Diagram of Indium (In).
Q1: What does the orbital diagram of indium show?
It shows how 49 electrons are distributed among indium’s atomic orbitals.
Q2: How many valence electrons are shown in the indium orbital diagram?
The indium orbital diagram shows three valence electrons (5s² 5p¹).
Q3: Why is there one unpaired electron in the orbital diagram of indium?
Because indium has one electron in the 5p orbital.
Q4: Is orbital notation for indium the same as electron configuration?
No, orbital notation visually shows electron spin and pairing, while configuration lists electron numbers only.






