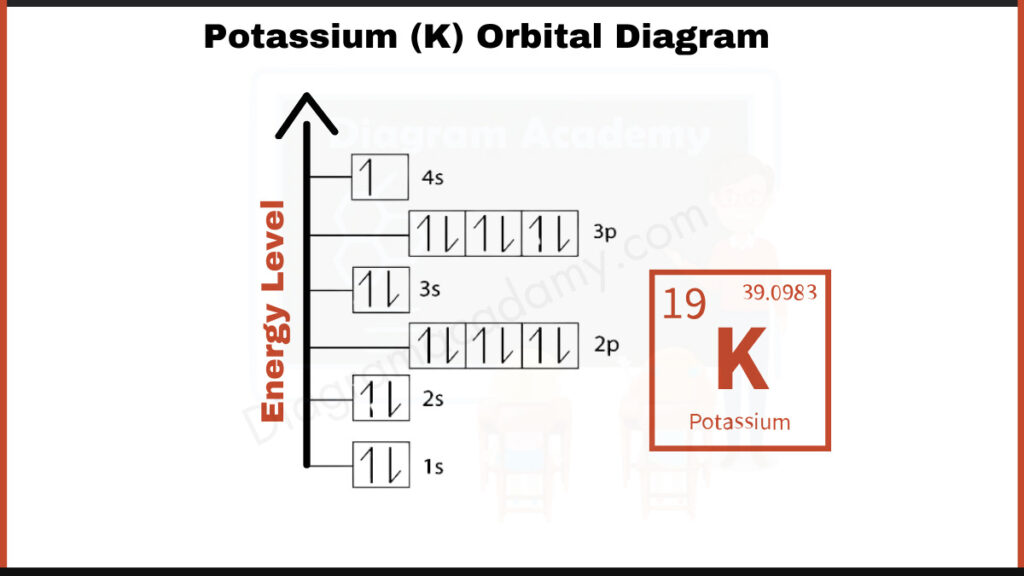
How to write the Orbital Diagram for Potassium?
Potassium (K) has 19 electrons. They fill shells in a specific order: [2, 8, 8, 1]. The first number, 2, represents the electrons in the innermost shell closest to the nucleus. The next two numbers, 8 and 8, indicate the electrons in the subsequent shells. The final number, 1, signifies the single electron in the outermost shell of potassium. This single electron in the outer shell plays a crucial role in how potassium behaves chemically, making it very reactive.