What Does the Hydrogen Electron Configuration Diagram Show?
A hydrogen electron configuration diagram visually represents how hydrogen’s single electron is arranged around its nucleus. Because hydrogen has only one electron, its diagram is the simplest of all elements and is often used to introduce atomic structure concepts.
Why Is Hydrogen’s Electronic Structure Unique?
Hydrogen is unique because it has only one proton and one electron. Unlike other elements, its electronic structure of hydrogen involves just a single orbital. This simplicity makes hydrogen ideal for explaining how electrons occupy energy levels.
What Is the Electron Configuration of Hydrogen?
The electron configuration of hydrogen is:
1s¹
This means hydrogen has one electron located in the first energy level (n = 1) within the s orbital. This configuration is the foundation for understanding atomic models and bonding behavior.
How Is Hydrogen Shown in an Electron Configuration Diagram?
In a hydrogen electron configuration diagram, the 1s orbital is shown as a single box containing one arrow. This arrow represents the lone electron and its spin. Because there are no other electrons, the diagram is clean and easy to interpret.
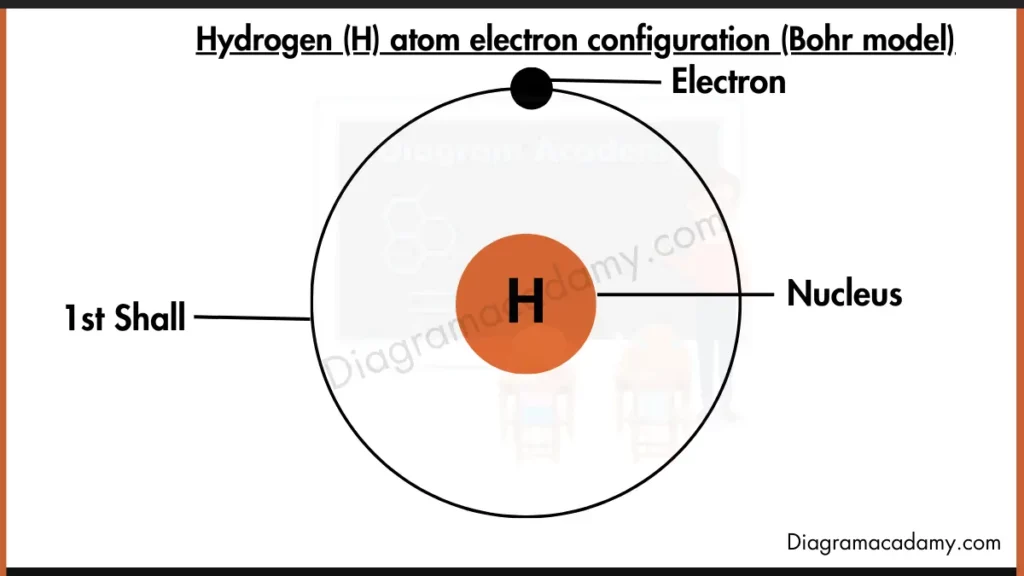
How Does the Hydrogen Bohr Model Represent Electrons?
The hydrogen Bohr model shows a nucleus at the center with one electron moving in a circular orbit around it. The Bohr model of hydrogen places the electron in the first energy level, making it one of the clearest examples of Bohr’s atomic theory.
How Do Hydrogen Electrons Determine Its Behavior?
The single electron in hydrogen plays a key role in chemical reactions. Hydrogen can lose, gain, or share this electron, which explains its ability to form bonds and exist in different forms such as H⁺ or H₂.
Why Is Hydrogen Configuration Important in Chemistry?
Understanding the hydrogen electron configuration helps students learn atomic theory, bonding, and periodic trends. Hydrogen is often the starting point for studying electron configurations and atomic models in chemistry and physics.
Frequently Asked FAQs
Here are answer to common question about Hydrogen Electron Configuration.
Q1: What is the electron configuration of hydrogen?
The electron configuration of hydrogen is 1s¹.
Q2: How many electrons does hydrogen have?
Hydrogen has one electron.
Q3: What does the hydrogen Bohr model show?
It shows one electron orbiting the nucleus in the first energy level.
Q4: Why is the hydrogen electron configuration so simple?
Because hydrogen has only one electron and one orbital involved.
Q5: Is the hydrogen electron configuration diagram useful for learning chemistry?
Yes, it is the simplest model used to introduce atomic structure concepts.






