What Does the Electron Configuration of Bohrium Explain?
The electron configuration of bohrium describes how electrons are distributed around the nucleus of a bohrium atom. For image-based learning, this configuration helps explain where electrons are located before converting the data into diagrams such as orbital or Bohr-style models.
Basic Atomic Details Needed to Understand Bohrium
Bohrium has the chemical symbol Bh and an atomic number of 107, meaning it contains 107 electrons. It is a synthetic, radioactive transition metal found in the d-block. These facts are essential for interpreting the electronic configuration of bohrium correctly.
What Is the Electronic Configuration of Bh?
The predicted ground-state electron configuration for bohrium is:
[Rn] 5f¹⁴ 6d⁵ 7s²
This configuration is theoretical because bohrium atoms exist for a very short time. Still, it is widely accepted and used in textbooks and diagram references.
How Is the Bohrium Electron Configuration Shown in Diagrams?
In diagram form, the bohrium electron configuration is displayed using orbital boxes or energy-level visuals. The inner electrons match the radon core, while the outer electrons occupy the 6d and 7s orbitals. This structure is clearly highlighted in configuration diagrams.
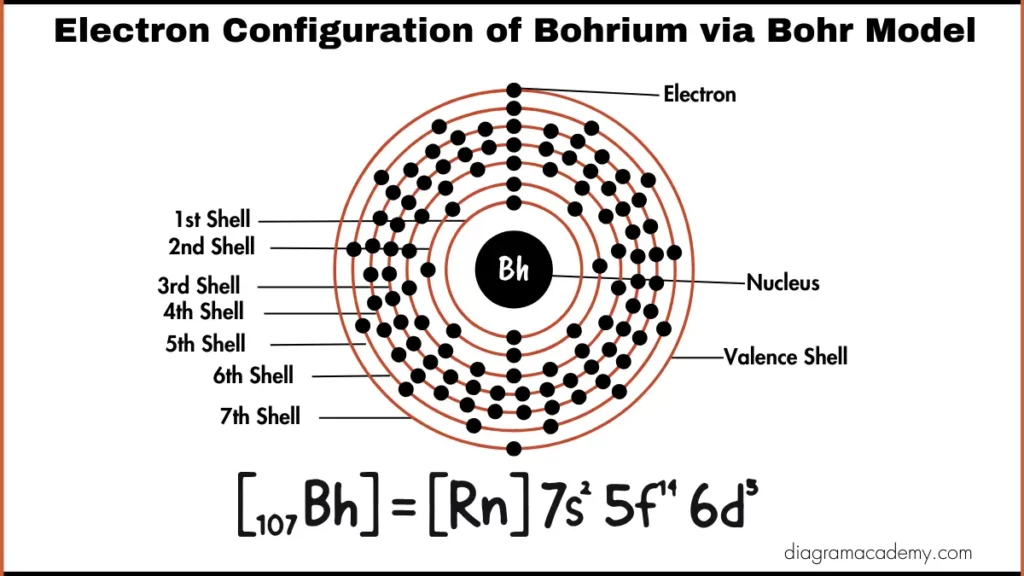
How Does the Bohrium Bohr Model Represent Electrons?
The bohrium Bohr model simplifies electron placement into energy levels rather than orbitals. While not fully accurate for heavy elements, the Bohr model of bohrium helps beginners visualize how electrons are arranged in shells around the nucleus.
Bohr Model vs Orbital Configuration for Bohrium
The Bohr model bohrium is useful for basic visualization, while the electron configuration of bohrium provides a more accurate quantum-based description. Both representations are commonly used on educational diagram websites for different learning levels.
Why Studying Bohrium’s Configuration Is Important
Understanding the electronic configuration of Bh helps explain periodic trends, transition-metal behavior, and advanced atomic theory. Bohrium is especially important in nuclear chemistry and superheavy element research.
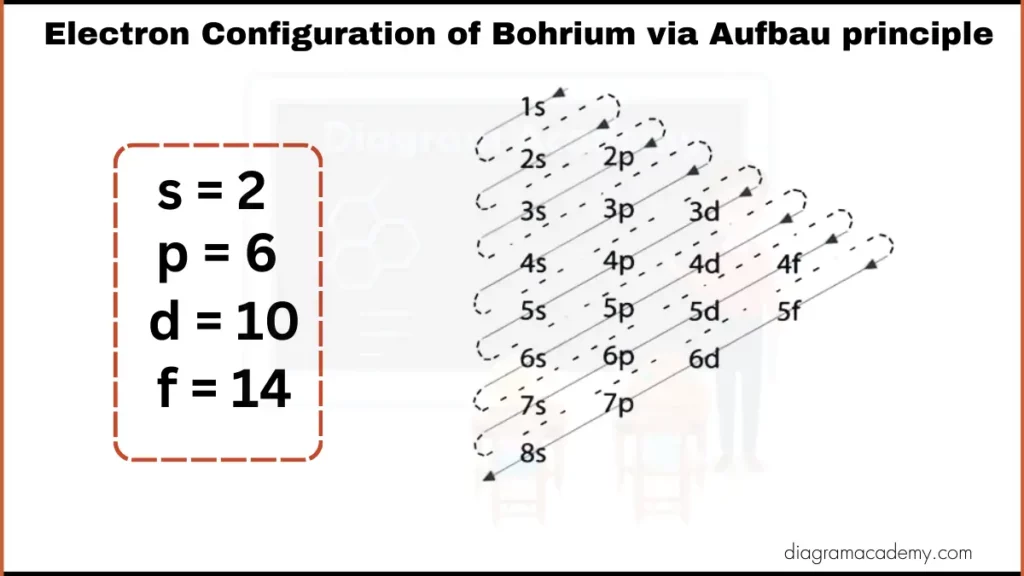
Electronic Configuration of Bohrium via Aufbau Principle
Frequently Asked FAQs
Here are answers to common questions about Bohrium Electron Configuration (Bh).
Q1: What is the electron configuration of bohrium?
The electron configuration of bohrium is [Rn] 5f¹⁴ 6d⁵ 7s².
Q2: Is the bh electron configuration experimentally confirmed?
No, it is predicted based on quantum mechanical models due to bohrium’s short half-life.
Q3: What does the bohrium Bohr model show?
It shows electrons arranged in energy levels around the nucleus in a simplified form.
Q4: Why is bohrium’s configuration considered theoretical?
Because bohrium atoms exist only briefly, direct measurement is extremely difficult.
Q5: Is the Bohr model of bohrium accurate?
It is useful for visualization but not fully accurate for heavy elements like bohrium.






