What Does the Bromine Bohr Diagram Represent?
The bromine Bohr diagram is a simplified visual model that shows how electrons are arranged in fixed energy levels around the nucleus of a bromine atom. The Bohr diagram of bromine uses circular shells to represent electron orbits, making it easier for students to understand atomic structure through images rather than complex orbital notation.
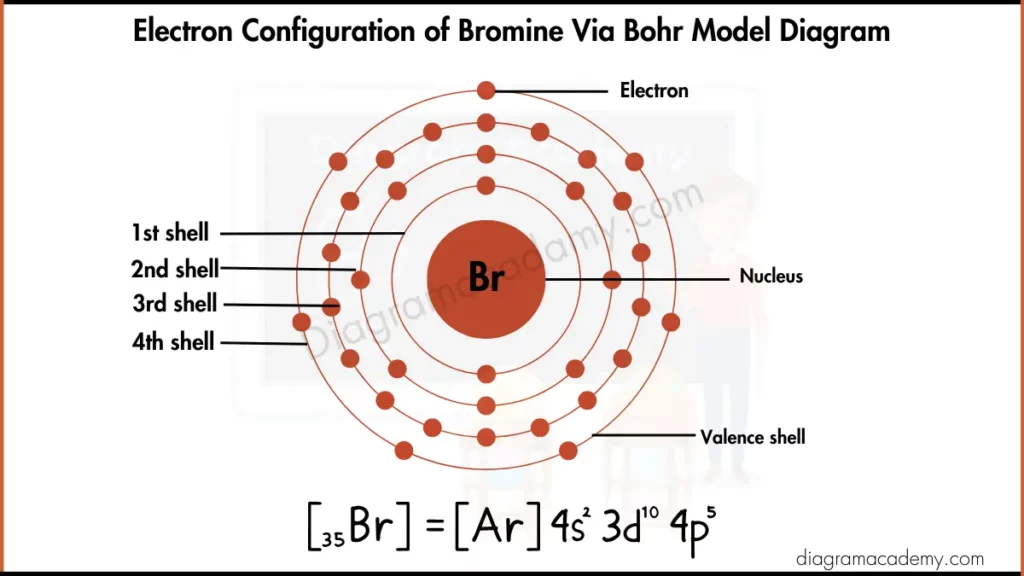
Electronic Configuration of Bromine via Bohr Model Diagram
Which Atomic Facts Are Used in the Bromine Bohr Model?
To draw the bromine Bohr model, basic atomic data is required. Bromine has the symbol Br and an atomic number of 35, meaning it contains 35 electrons. These electrons are placed into energy levels according to the Bohr theory, forming the foundation of the Bohr diagram for bromine.
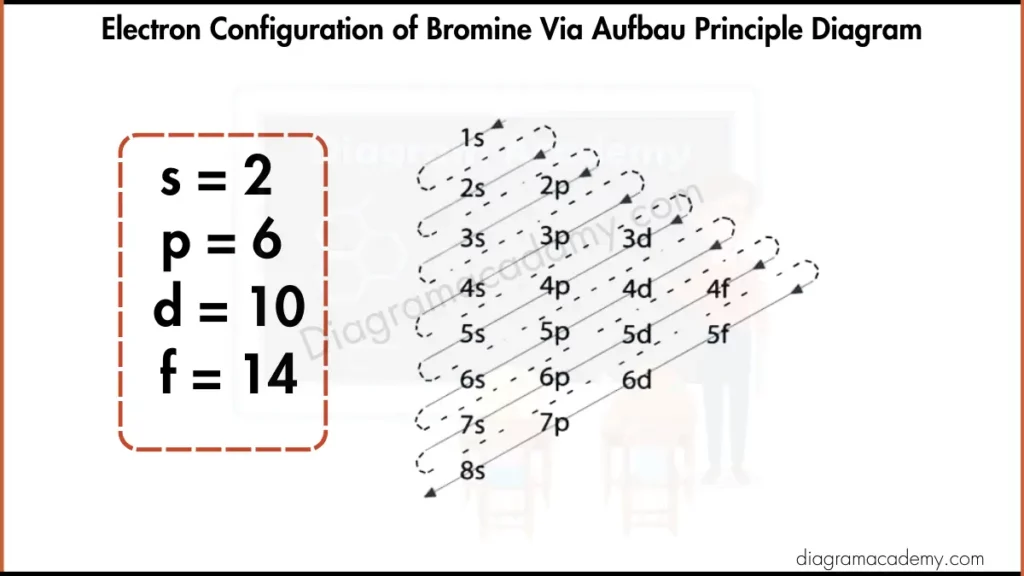
Electronic Configuration of Bromine via Aufbau Principle Diagram
How Are Electrons Arranged in the Bohr Diagram of Bromine?
In the Bohr diagram bromine, electrons are distributed across four energy levels. The electron arrangement is 2, 8, 18, 7, starting from the shell closest to the nucleus. This clear shell-based structure is why the bromine Bohr diagram is commonly used in introductory chemistry and physics.
How Does the Bohr Diagram for Bromine Compare with Electron Configuration?
The electron configuration of bromine provides detailed orbital information, while the Bohr diagram of bromine simplifies this data into shells. Both models describe the same electrons, but the Bohr model focuses on energy levels, making it ideal for visual learning and diagram-based explanations.
What Is the Bromine Bohr Rutherford Diagram?
The bromine Bohr Rutherford diagram combines Rutherford’s nuclear model with Bohr’s energy levels. It shows a central nucleus containing protons and neutrons, surrounded by electron shells. This diagram helps learners visualize both nuclear structure and electron distribution at once.
Why Is the Bohr Model of Bromine Important for Learning?
The Bohr model bromine is important because it clearly shows bromine’s seven valence electrons, explaining its chemical reactivity. It is widely used in school-level education, exams, and image-based chemistry resources to simplify atomic concepts.
Frequently Asked FAQs
Here are the answer to common question about Bromine Bohr Diagram.
Q1: What does the bromine Bohr diagram show?
It shows the distribution of 35 electrons in fixed energy levels around the nucleus.
Q2: How many shells are shown in the Bohr diagram of bromine?
The Bohr diagram of bromine shows four electron shells.
Q3: How many valence electrons are in the bromine Bohr model?
The bromine Bohr model shows seven valence electrons in the outermost shell.
Q4: Is the Bohr diagram for bromine the same as electron configuration?
No, the Bohr diagram simplifies electrons into shells, while electron configuration shows orbitals.
Q5: Why is the bromine Bohr Rutherford diagram useful?
It visually combines the nucleus and electron shells, making atomic structure easier to understand.






