What Does an Aufbau Diagram for Chlorine Show?
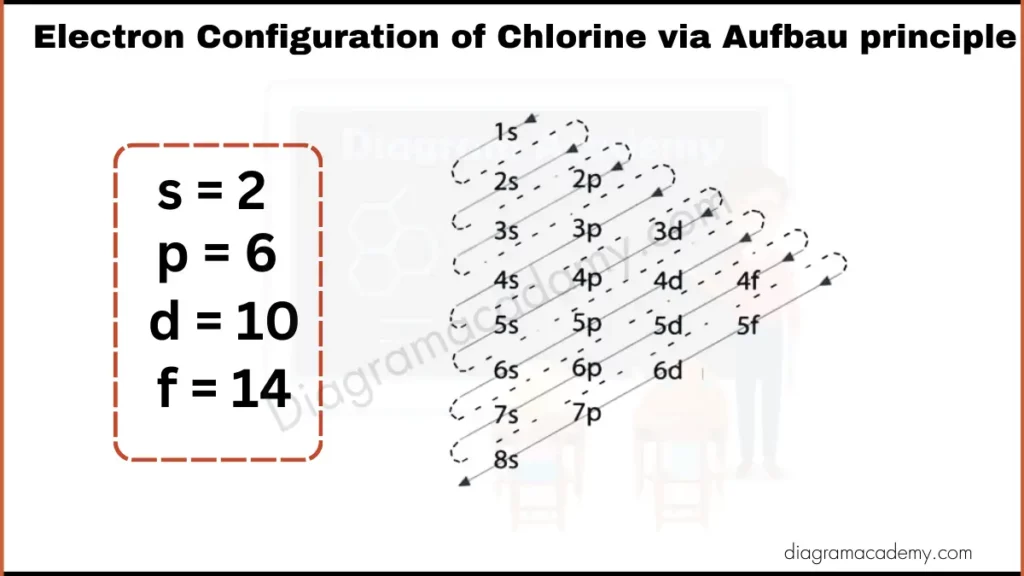
An Aufbau diagram for chlorine explains how electrons are filled into atomic orbitals step by step. It follows the Aufbau principle, which states that electrons enter the lowest-energy orbitals first before moving to higher ones.
For chlorine (atomic number 17), the diagram visually represents how its 17 electrons are arranged across different subshells like s and p.
This diagram is useful for understanding electron pairing, orbital order, and valence electrons, which are important for predicting chemical behavior.
How Are Chlorine’s Electrons Filled According to the Aufbau Principle?
Chlorine’s electrons are added in a specific sequence based on increasing orbital energy:
- Lower-energy orbitals fill first
- Each orbital can hold a maximum of two electrons
- Electrons occupy separate orbitals before pairing (Hund’s rule)
Following this order, chlorine’s electrons fill up to the 3p subshell, where it has five electrons, leaving one spot unpaired.
What Is the Orbital Filling Order for Chlorine?
The orbital filling sequence for chlorine follows this pattern:
- 1s orbital fills first
- Then 2s and 2p orbitals
- Followed by 3s and finally 3p orbitals
This order explains why chlorine ends with five electrons in the 3p orbital, making it highly reactive and eager to gain one more electron.
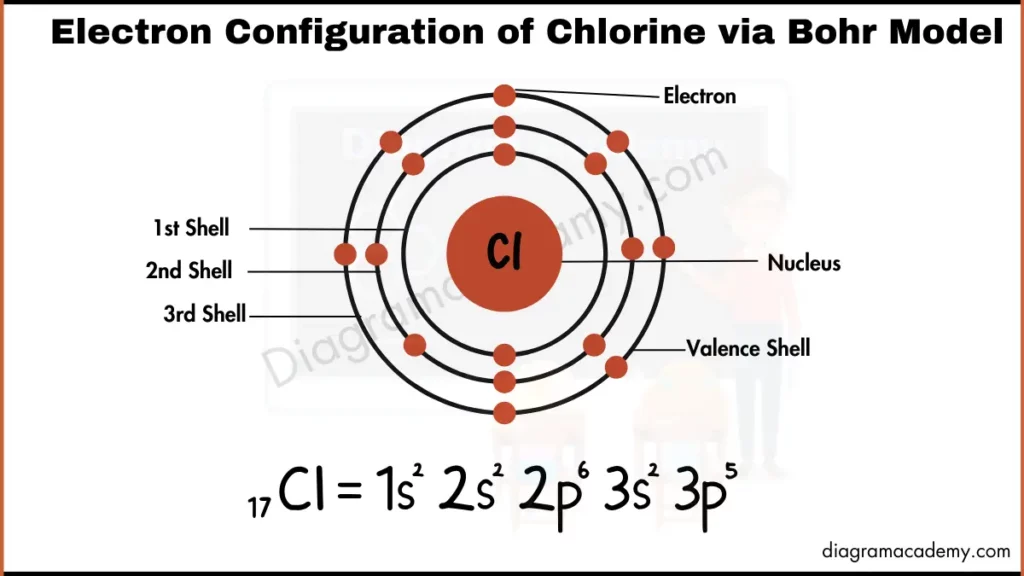
Diagram of Electronic Configuration of Chlorine via Bohr Model
What Is the Electron Configuration Represented by the Aufbau Diagram?
The Aufbau diagram visually represents chlorine’s electron arrangement as:
1s² 2s² 2p⁶ 3s² 3p⁵
This configuration shows that chlorine has:
- Three occupied energy levels
- Seven electrons in its outermost shell
- One unpaired electron that plays a key role in bonding
How Does the Aufbau Diagram Differ from the Bohr Diagram of Chlorine?
While both diagrams describe electron arrangement, they focus on different ideas:
- Aufbau diagram shows electrons in orbitals using arrows and boxes
- Bohr diagram shows electrons in circular energy levels around the nucleus
The Aufbau model is more detailed and accurate for explaining bonding, magnetism, and orbital overlap, especially for chemistry students.
Why Is the Aufbau Diagram Important for Understanding Chlorine?
The Aufbau diagram helps explain why chlorine:
- Forms one bond easily
- Has strong electronegativity
- Commonly gains one electron to form Cl⁻
It also helps students understand valence electrons, reactivity, and orbital structure, which are not clearly shown in simpler atomic models.
How Can Students Easily Draw an Aufbau Diagram for Chlorine?
To draw it correctly:
- Write orbitals in energy order
- Add electrons one by one using arrows
- Pair electrons only after each orbital has one
This method ensures the diagram follows Aufbau, Pauli exclusion, and Hund’s rules accurately.
Diagram of Electronic Configuration of Chlorine via Aufbau Principle
Frequently Asked FAQs About Chlorine Electron Configuration
What is the electron configuration of chlorine?
Chlorine has 17 electrons arranged as 1s² 2s² 2p⁶ 3s² 3p⁵, with seven electrons in its outer shell.
What does the Aufbau diagram show for chlorine?
It shows how chlorine’s electrons fill orbitals from lower to higher energy, ending in the 3p⁵ subshell.
How many valence electrons does chlorine have?
Chlorine has seven valence electrons, which explains its strong tendency to gain one electron.
Why does chlorine have an unpaired electron?
Because the 3p subshell is not completely filled, one electron remains unpaired according to Hund’s rule.
Is the Aufbau diagram different from the Bohr model?
Yes. The Aufbau diagram shows orbital filling and electron spin, while the Bohr model only shows electrons in shells.
Why is chlorine highly reactive?
Chlorine is reactive because it needs just one more electron to achieve a stable outer shell.






