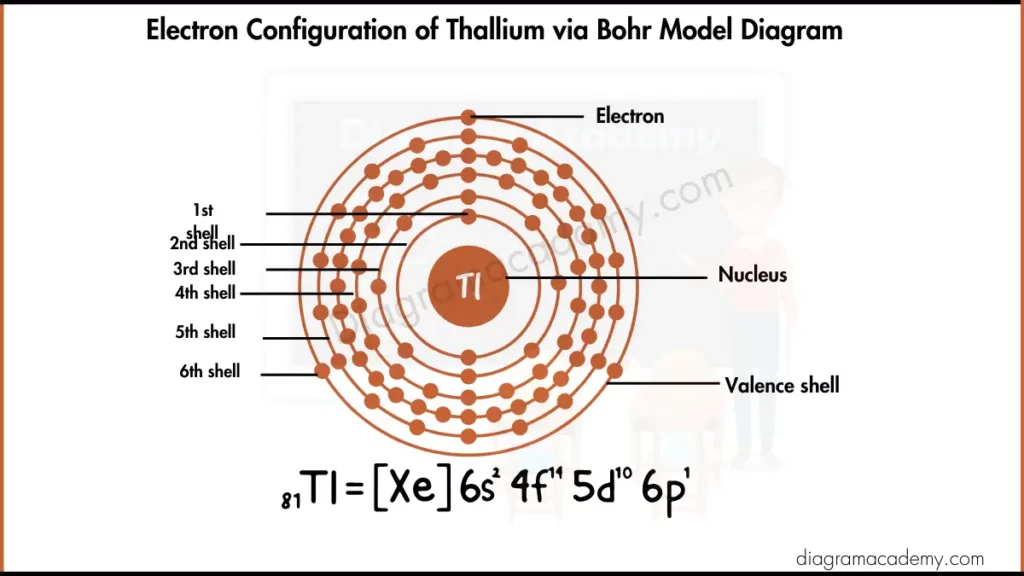
What Does the Thallium Bohr Model Show?
The thallium Bohr model is a visual representation that shows how electrons are distributed in fixed energy levels around the nucleus of a thallium atom. The Bohr model of thallium uses circular shells to make atomic structure easy to understand, especially for image-based learning.
Which Atomic Details Are Needed for the Bohr Model of Thallium?
Thallium has the chemical symbol Tl and an atomic number of 81. This means thallium contains 81 protons in its nucleus and 81 electrons surrounding it. These values are essential for drawing an accurate bohr model for thallium.
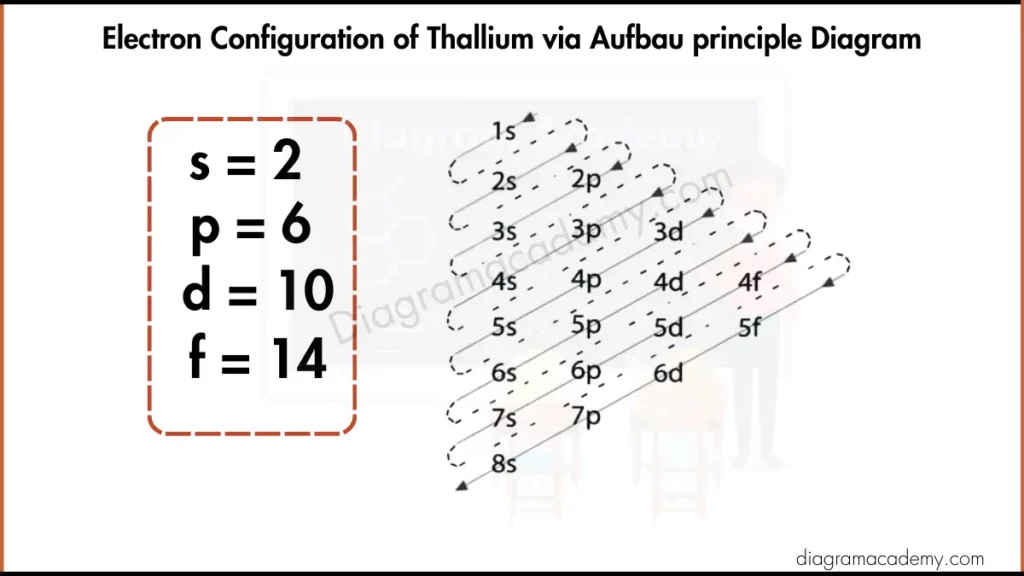
What Is the Electronic Configuration of Thallium?
The electronic configuration of thallium is:
[Xe] 4f¹⁴ 5d¹⁰ 6s² 6p¹
This configuration explains how electrons fill orbitals before being grouped into shells in the thallium electron configuration diagram.
How Are Electrons Arranged in the Bohr Model for Thallium?
In the bohr model of thallium, electrons are arranged into energy levels as:
2, 8, 18, 32, 18, 3
The outermost shell contains three electrons, which are the valence electrons. This shell structure is clearly shown in the thallium Bohr model.
Electronic Configuration of Thallium Diagram via Bohr Model
How Does the Bohr Model Relate to Thallium Electron Configuration?
The thallium electronic configuration provides detailed orbital information, while the Bohr model of thallium simplifies this into shell-based rings. Both describe the same electrons but at different levels of detail, making them useful for different learning stages.
How Many Protons and Electrons Does Thallium Have?
Thallium has 81 protons and 81 electrons in a neutral atom. The number of protons determines the element’s identity, while the electron arrangement defines its chemical behavior.
Why Is the Thallium Bohr Model Important for Learning?
The Bohr model for thallium helps students understand valence electrons, bonding behavior, and periodic trends. It is commonly used in school-level chemistry, exams, and diagram-based educational websites.
Electronic Configuration of Thallium Diagram via Aufbau Principle
Frequently Asked FAQs
Here are Answer to common question about Electronic Configuration of Thallium.
Q1: What does the thallium Bohr model show?
It shows how 81 electrons are arranged in energy levels around the nucleus.
Q2: How many valence electrons are in the Bohr model of thallium?
The Bohr model of thallium shows three valence electrons in the outermost shell.
Q3: What is the electronic configuration of thallium?
The electronic configuration of thallium is [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p¹.
Q4: How many protons does thallium have?
Thallium has 81 protons.
Q5: Is the Bohr model of thallium accurate for advanced chemistry?
It is useful for visualization, but orbital diagrams give more detailed information.






