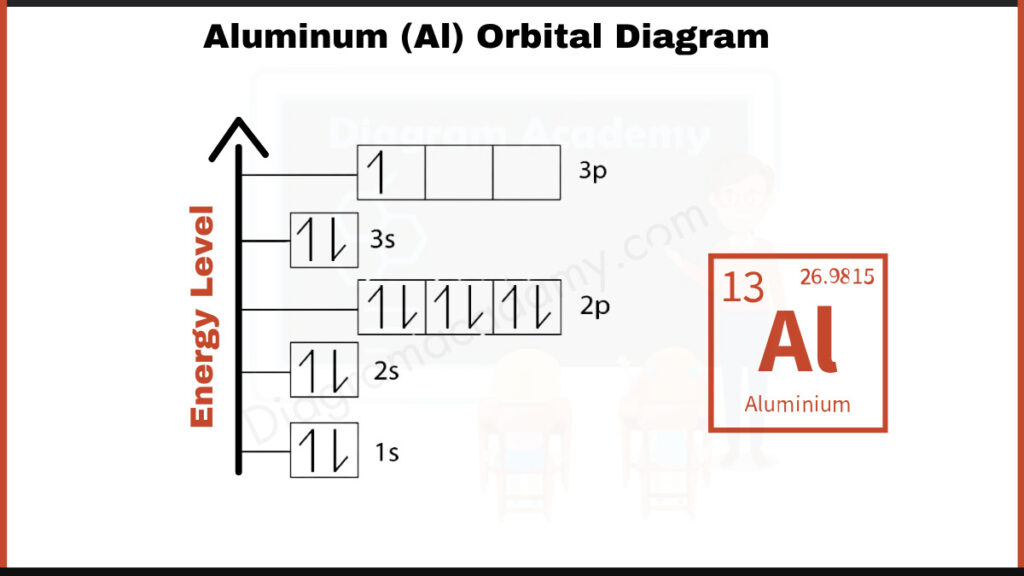
How to write the Orbital Diagram for Aluminium?
Aluminum (Al) has 13 electrons. They fill shells in a specific order: [2, 8, 3]. The first number, 2, represents the electrons in the first shell closest to the nucleus. The second number, 8, represents the electrons in the second shell. The last number, 3, represents the electrons in the outermost shell. This way of representing electron configuration shows how aluminum fills its shells before moving on to the next.