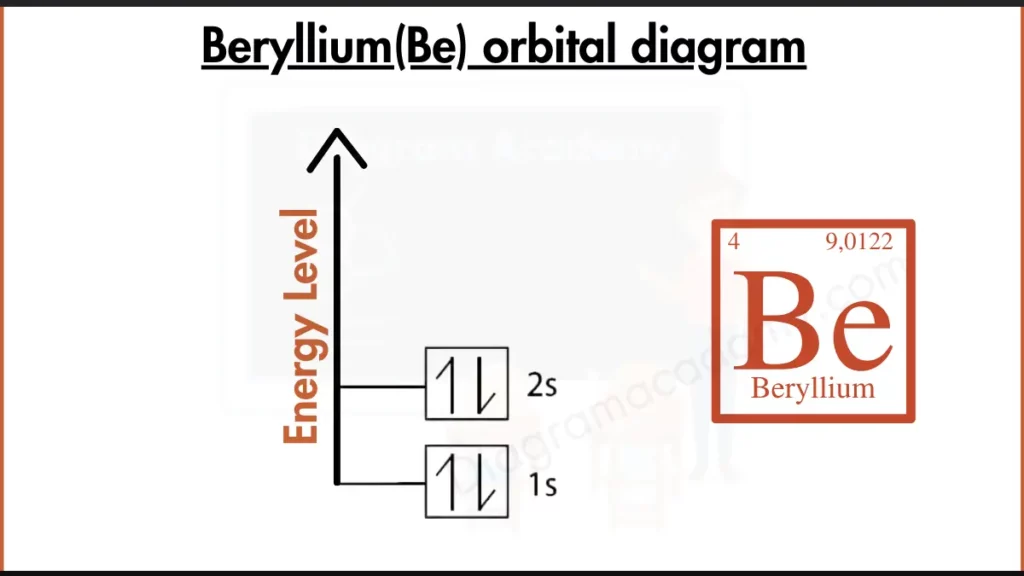
How to Write the Orbital Diagram for Beryllium?
Beryllium (Be) has 4 electrons, fills its inner shell (1s²) and places both remaining electrons in the second energy level’s s subshell (2s²). This complete outer s subshell gives beryllium a stable electronic configuration. It contributes to its relative inertness compared to neighboring elements.