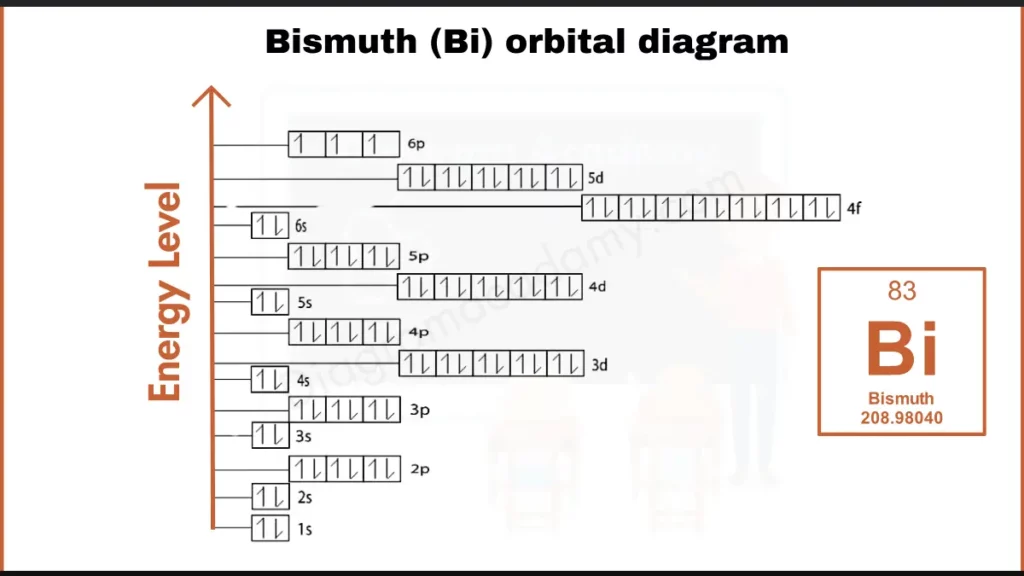
How to write Orbital Diagram of Bismuth?
Bismuth (Bi), with 83 electrons, possesses an intriguing configuration ([Xe] 4f¹⁴ 5d³ 6s²). Unlike its group members, Bismuth hasa filled outermost shell (6s²) alongside partially filled inner orbitals (5d³). This unique arrangement influences Bismuth’s metallic properties and bonding behavior.