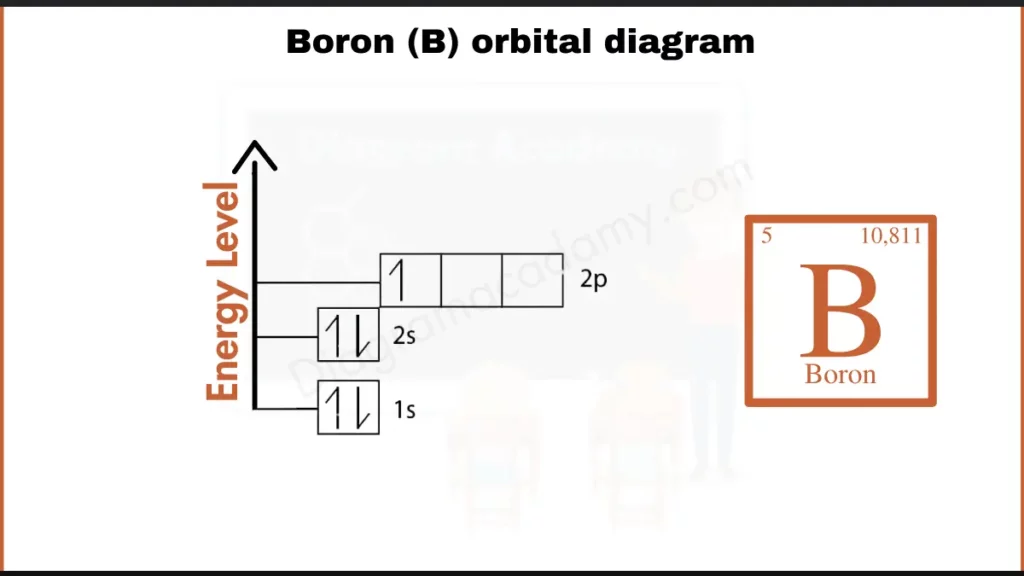
How to Write the Orbital Diagram for Boron?
Boron (B), with 5 electrons, has the configuration 1s²2s²2p¹. This fills its inner shells (1s and 2s) and places just 1 electron in its outermost p-orbital (2p¹). This single valence electron makes boron interesting – it can’t achieve a full outer shell by itself, leading to its unique bonding behavior.