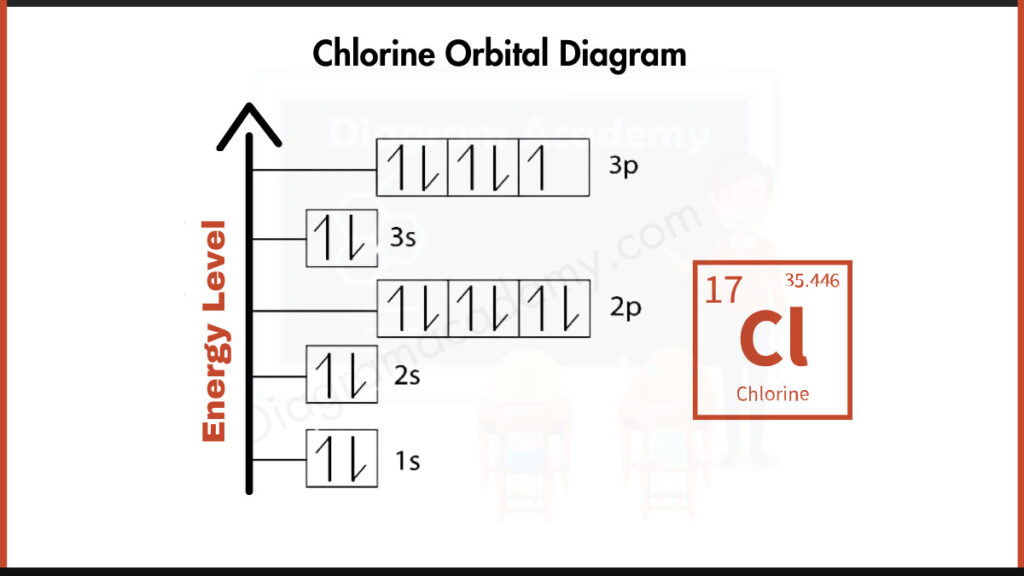
How to write the Orbital Diagram for Chlorine?
Chlorine (Cl) has 17 electrons. They fill shells around the nucleus following a specific order. In simpler terms, imagine these electrons filling buckets layer by layer. Chlorine has 2 electrons in the first layer (1s), 8 in the second (2s and 2p), and the remaining 7 electrons fill the third layer (3s and 3p).