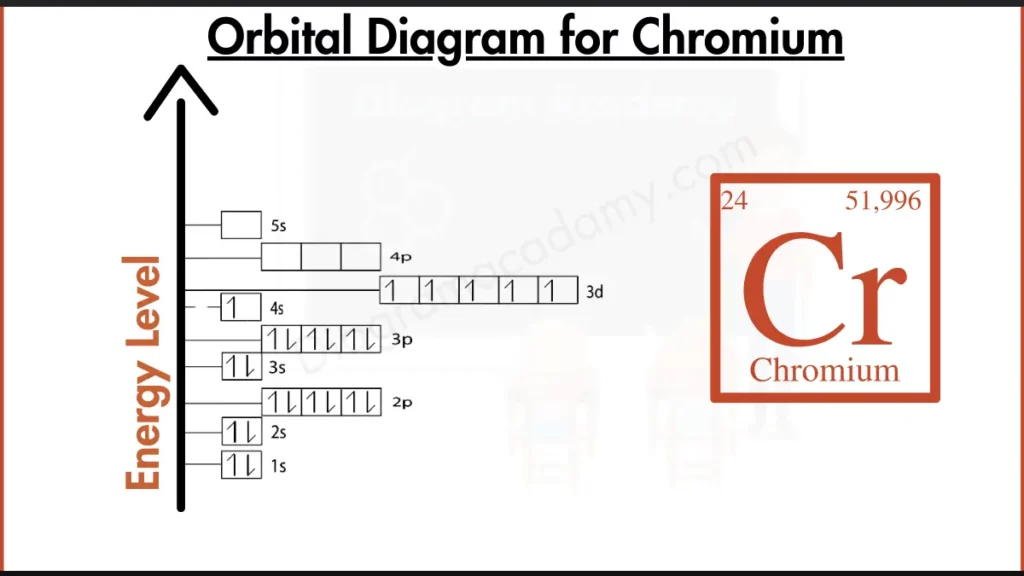
How to write the Orbital Diagram for Chromium?
Chromium (Cr) has 24 electrons filling its shells. The arrangement is typical for 3d transition metals: with 2 electrons in the first shell, 8 in the second, 13 in the third, and the remaining 1 electron in the fourth shell.