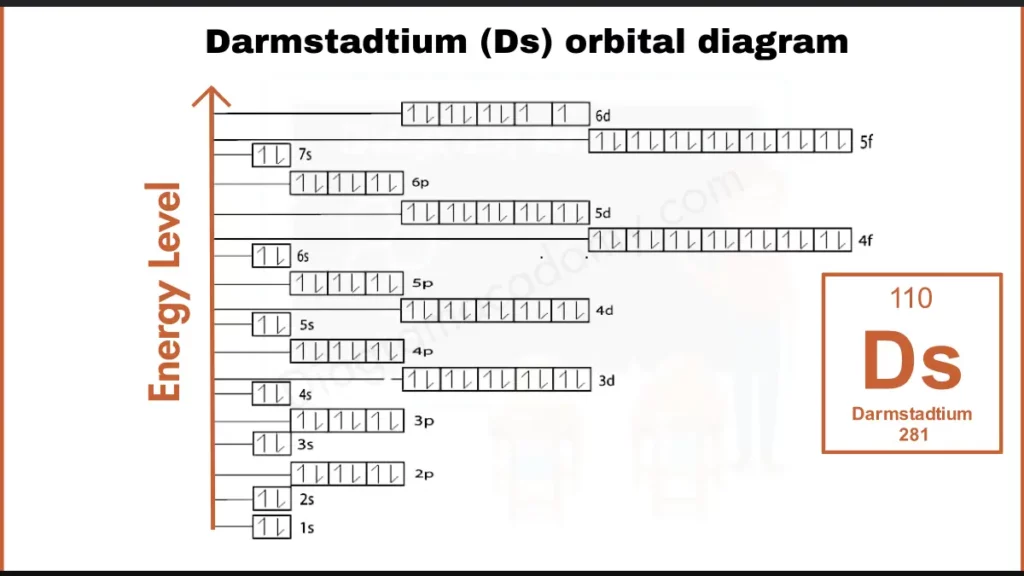
How to write Orbital Diagram of Darmstadtium?
Darmstadtium (Ds), with 110 electrons, has a complex configuration: [Rn] 5f¹⁴ 7s² 6d⁸ .The key to its bonding behavior lies in the outermost 6d subshell, which holds an unusual set of eight electrons (6d⁸). This unique configuration is believed to influence Darmstadtium’s interactions with other elements.