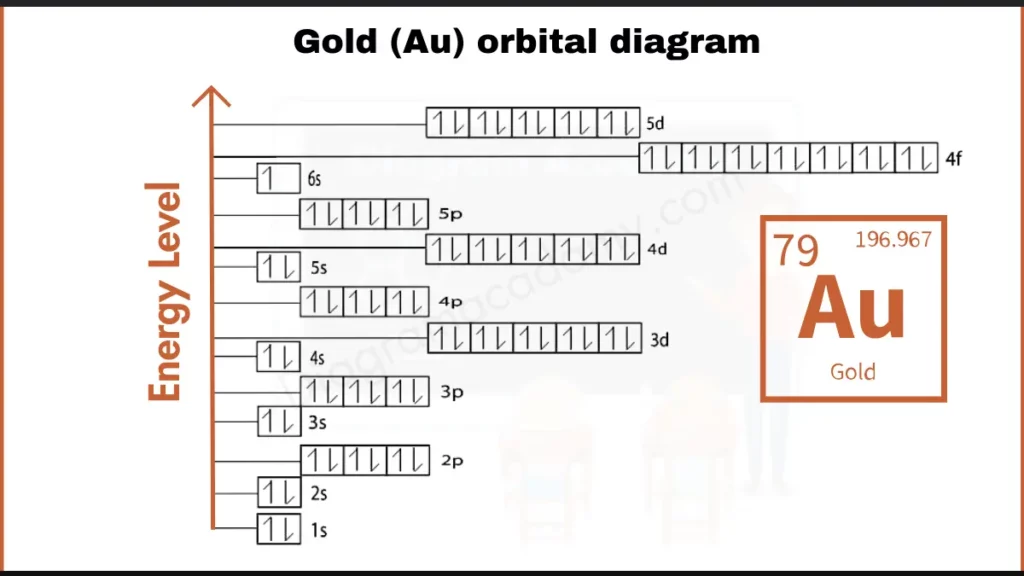
How to write Orbital Diagram of Gold?
Gold (Au) boasts 79 electrons. Its configuration, [Xe] 4f¹⁴ 5d¹⁰ 6s¹, reveals a twist. It has a full inner shell (5d¹⁰) and a single electron in the outermost shell (6s¹). This unique setup influences Gold’s metallic nature and remarkable resistance to tarnishing.