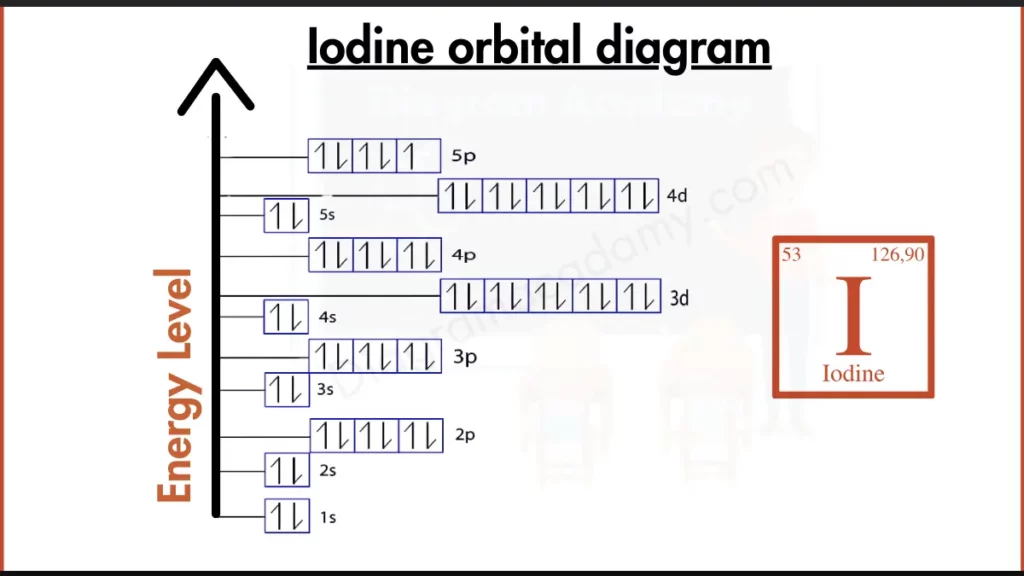
How to write the Orbital Diagram for Iodine?
Iodine (I) joins the club of reactive halogens. Its outer shell holds seven electrons, written as [Kr] 4d¹⁰ 5s² 5p⁵. This configuration, with a partially filled 5p subshell, makes Iodine very reactive. Unlike the stable noble gases with packed outer shells, Iodine readily seeks to gain one electron to achieve a full outer shell and become more stable, forming the negatively charged iodide ion (I⁻)