What Is the Orbital Diagram for Krypton?
The orbital diagram for krypton is a visual model that shows how electrons are arranged in the atomic orbitals of a krypton atom. The krypton orbital diagram uses boxes and arrows to represent orbitals and electron spins, making it easier to understand electron distribution compared to text-only configurations.
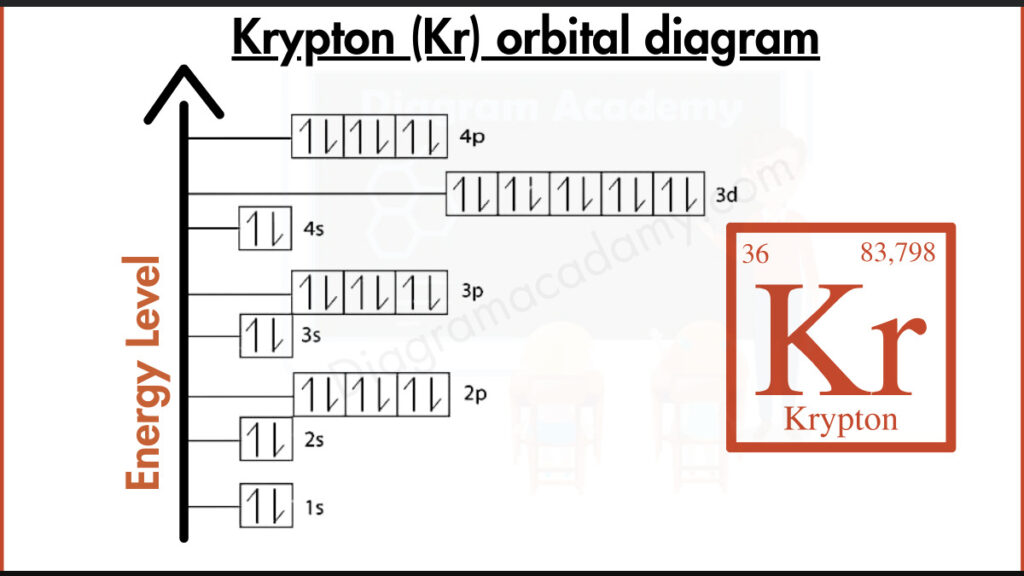
What Atomic Information Is Needed for the Krypton Orbital Diagram?
To understand the orbital diagram of krypton, basic atomic details are required. Krypton has the symbol Kr and an atomic number of 36, meaning it contains 36 electrons. It is a period-4 noble gas and belongs to the p-block, which strongly influences the orbital diagram krypton.
How Does Electron Configuration Explain the Orbital Diagram of Krypton?
The electron configuration of krypton is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
This complete configuration explains why all orbitals in the orbital diagram for Kr are fully filled, resulting in a stable atomic structure.
How Is the Orbital Diagram for Kr Filled Step by Step?
In the kr orbital diagram, electrons fill orbitals from lower to higher energy following the Aufbau principle. The 4s orbital fills before the 3d orbitals, and finally the 4p orbitals are filled. Each orbital contains paired electrons, clearly shown in the orbital diagram kr.
How Are Energy Levels Shown in the Orbital Diagram Kr?
The orbital diagram of krypton is arranged vertically to represent increasing energy levels. Lower-energy orbitals appear at the bottom, while higher-energy orbitals like 4p appear at the top. This layout helps viewers quickly read the orbital diagram for krypton.
What Is Orbital Notation for Krypton?
Orbital notation for krypton uses paired arrows in every orbital to represent opposite electron spins. Because krypton has no unpaired electrons, its krypton orbital notation clearly reflects its chemical stability.
Why Is the Orbital Diagram for Krypton Important?
The orbital diagram for krypton explains why krypton is chemically inert. It is widely used in chemistry education, exams, and diagram-based learning platforms to demonstrate noble gas stability.
Frequently Asked FAQs
Here are the answer to common question about Orbital diagram of Krypton (Kr).
Q1: What does the orbital diagram for krypton show?
It shows how 36 electrons are fully paired in krypton’s atomic orbitals.
Q2: Why are there no unpaired electrons in the krypton orbital diagram?
Because all orbitals are completely filled, making krypton stable.
Q3: Where are the highest energy electrons shown in the orbital diagram of krypton?
They appear in the 4p orbitals at the top of the diagram.
Q4: Is orbital notation for krypton different from electron configuration?
Yes, orbital notation for krypton visually shows electron pairing and spin.
Q5: Why is the orbital diagram for Kr important in chemistry?
It explains krypton’s lack of reactivity and noble gas behavior.






