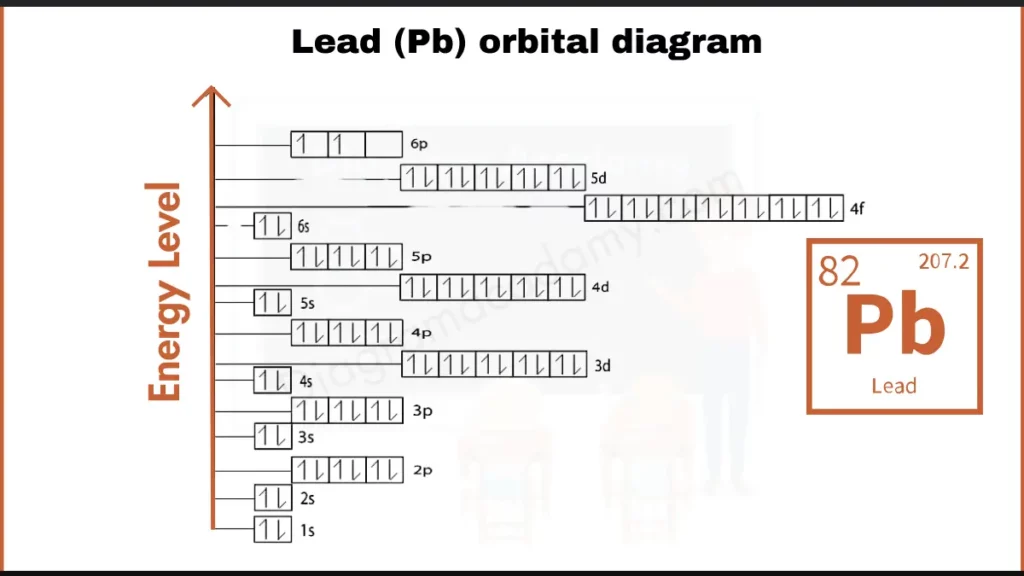
How to write Orbital Diagram of Lead?
Lead (Pb) possess 82 electrons. Unlike some elements, it fills its inner shells completely ([Xe] 4f¹⁴ 5d¹⁰ 6s². It fill shells in brackets as [Xe]) before adding to the outermost 6p subshell (6p²). This complete inner shell setup influences Lead’s high density and easy shaping (malleability).