What Does a Meitnerium Orbital Diagram Represent?
A meitnerium orbital diagram visually shows how electrons are arranged in the atomic orbitals of the element meitnerium. Instead of listing electrons in text form, the diagram uses orbital boxes and arrows to clearly represent electron occupancy and spin. This makes the electron configuration of meitnerium easier to understand through visuals.
Key Atomic Facts Needed Before Drawing Meitnerium Orbitals.
Meitnerium has the symbol Mt and an atomic number of 109, meaning it contains 109 electrons. It is a synthetic, superheavy element classified in the d-block. These details are essential when interpreting a meitnerium drawing or orbital-based diagram.
How Electron Configuration Determines the Meitnerium Diagram?
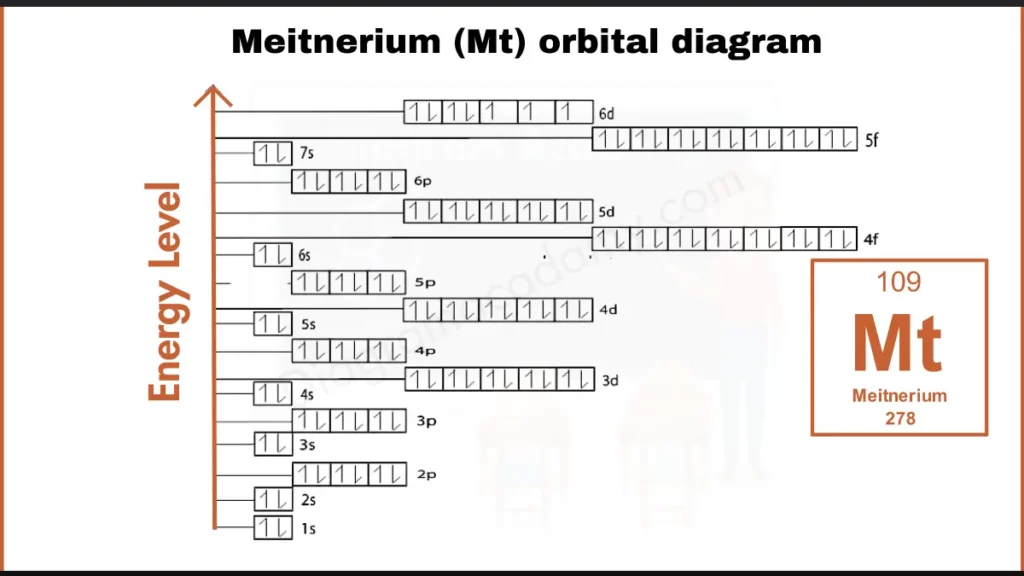
The predicted ground-state electron configuration of meitnerium is:
[Rn] 5f¹⁴ 6d⁷ 7s²
This configuration directly defines how orbitals are filled in the meitnerium orbital diagram, especially the involvement of 6d and 7s orbitals.
How Electrons Are Placed in the Meitnerium Orbital Diagram?
In the orbital diagram of meitnerium, electrons fill orbitals starting from lower energy levels. The 7s orbital fills before the 6d orbitals. Within the 6d subshell, electrons occupy separate orbitals first before pairing, following Hund’s rule. This filling order is visually clear in a properly labeled electron configuration diagram.
How Energy Levels and Orbitals Appear in the Diagram?
The diagram is arranged vertically to show increasing energy. Core orbitals are shown lower, while valence orbitals appear near the top. This layout helps viewers understand how meitnerium orbitals relate to one another energetically.
How Meitnerium Orbital Notation Is Interpreted?
Orbital notation uses arrows to represent electron spin within each orbital. In meitnerium, several unpaired electrons are expected in the d orbitals, which can be identified directly from the notation used in the diagram.
Why Orbital Diagrams of Meitnerium Matter?
Although meitnerium has no everyday applications, its orbital diagram is important for studying periodic trends, relativistic effects, and advanced atomic structure in heavy elements
Frequently Asked FAQs
Here are the answer to common question about Meitnerium Orbital Diagram (Mt).
Q1: What does a meitnerium orbital diagram show?
It shows how 109 electrons are arranged in meitnerium’s atomic orbitals.
Q2: Is the electron configuration of meitnerium confirmed experimentally?
No, it is predicted based on quantum models due to the element’s short half-life.
Q3: Which orbitals are involved in the outer shell of meitnerium?
The outer electrons mainly occupy the 7s and 6d orbitals.
Q4: Why are meitnerium diagrams mostly theoretical?
Because meitnerium atoms exist only briefly, making direct measurement difficult.
Q5: Is a meitnerium drawing useful for learning chemistry?
Yes, it helps students understand orbital filling trends in superheavy elements.






