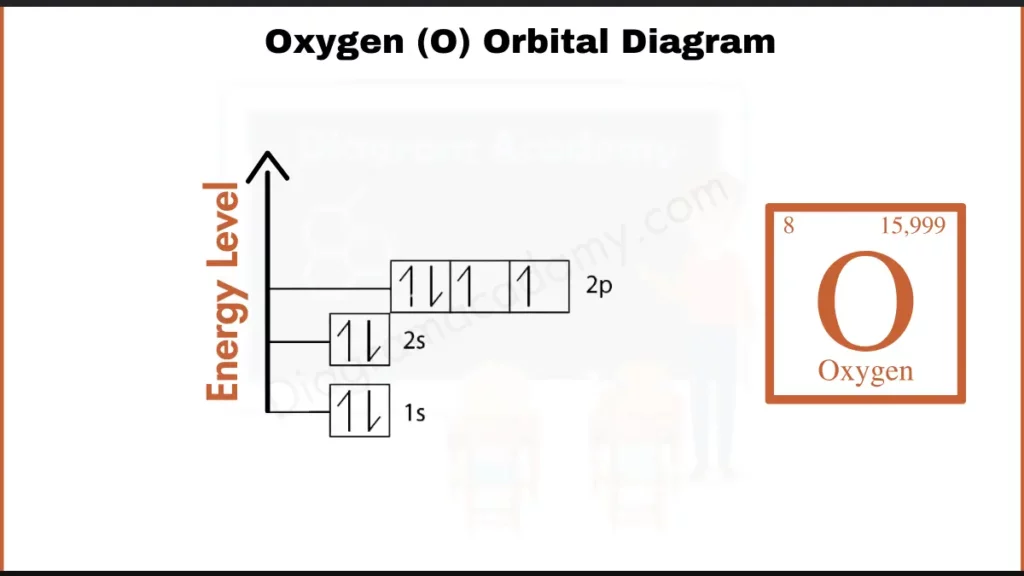
How to Write the Orbital Diagram for Oxygen?
Oxygen (O), with 8 electrons, fills its first two shells (1s²2s²) and puts 4 electrons in its outermost p-orbital (2p⁴). This incomplete p-orbital makes oxygen reactive. it needs two electrons to fill its outermost p subshell.