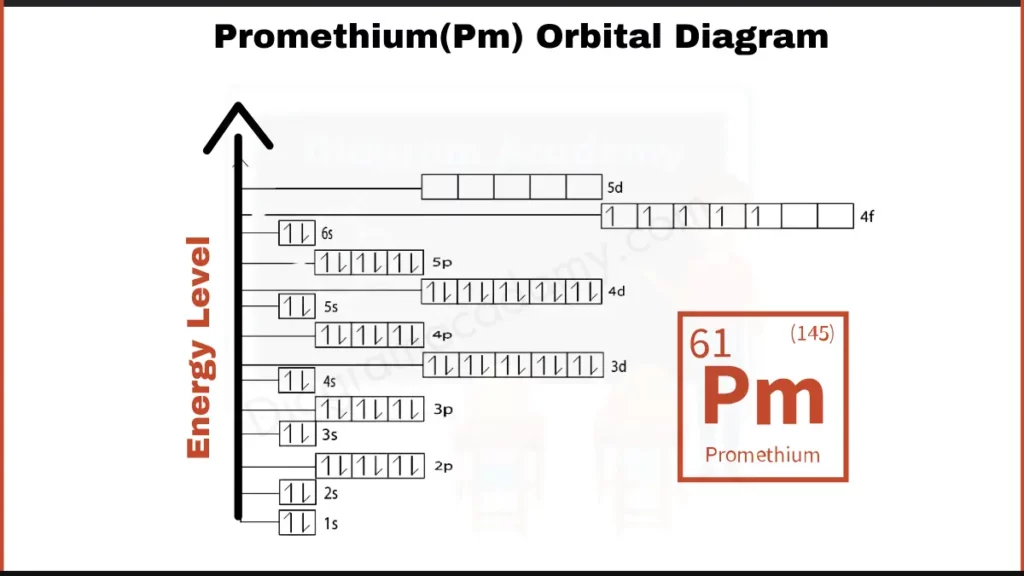
How to write the Orbital Diagram for Promethium?
Promethium (Pm) packs 61 electrons around its nucleus. Unlike most lanthanides, its electron arrangement has a twist. It has 61 electrons, but among them, there are 21 electrons filling an incomplete f-orbital (4f subshell). The remaining electrons reside in the usual shells. This unique configuration sets Promethium apart from other lanthanides.