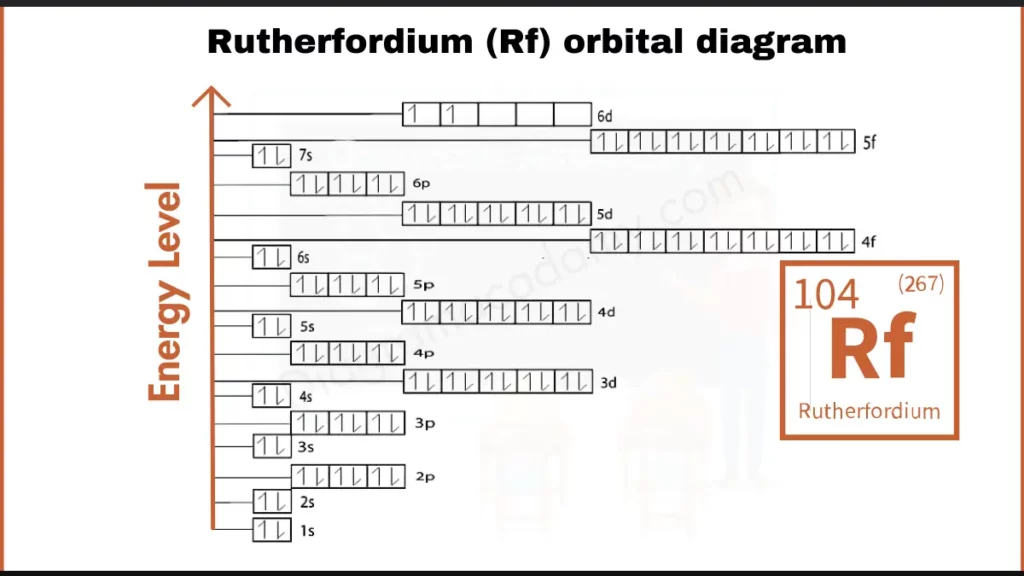
How to write Orbital Diagram of Rutherfordium?
Rutherfordium (Rf), with 104 electrons, has a complex configuration: [Rn] 5f¹⁴ 6d² 7s². The key to its bonding behavior lies in the partially filled 6d subshell, holding just two electrons (6d²). This incomplete configuration, unlike the stable outer shells of some heavier elements, is believed to influence Rutherfordium’s unique bonding properties.