What Does an Orbital Diagram Represent for Scandium?
The scandium orbital diagram visually shows how electrons are arranged in the orbitals of a scandium atom. Instead of a simple electron list, the orbital diagram for scandium uses boxes for orbitals and arrows for electrons, making it easier to understand electron pairing and energy levels.
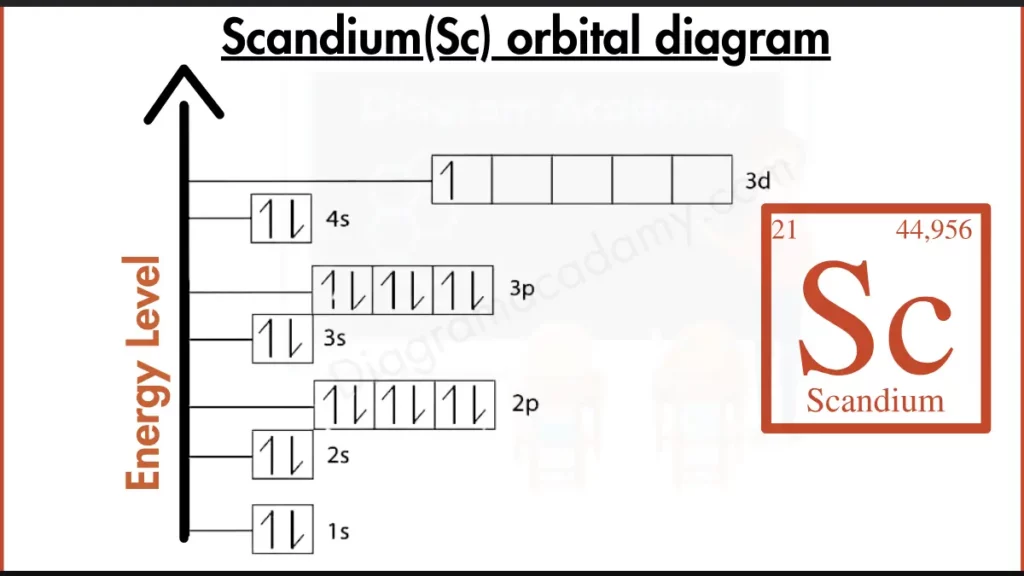
Which Atomic Details Shape the Scandium Orbital Diagram?
Scandium has the atomic symbol Sc and atomic number 21, meaning it contains 21 electrons. It is a transition metal in period 4. These atomic characteristics explain why both s and d orbitals appear in the orbital diagram of scandium.
How Is Electron Configuration Converted into an Orbital Diagram?
The electron configuration of scandium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹
This configuration is translated into the sc orbital diagram by placing electrons into orbitals in order of increasing energy, using arrows to show their spins.
How Does the Orbital Filling Pattern Work in Scandium?
In the orbital diagram for Sc, electrons fill the lower-energy orbitals first, following the Aufbau principle. The 4s orbital fills before the 3d orbital, resulting in one unpaired electron in the 3d subshell. This sequence is clearly shown in the orbital diagram scandium.
How Are s and d Orbitals Displayed in the Sc Orbital Diagram?
The orbital diagram of Sc shows filled s orbitals and a partially filled d orbital. The 3d subshell contains one electron, which is important for understanding scandium’s metallic and bonding behavior.
What Does Orbital Notation of Scandium Look Like?
Scandium orbital notation uses arrows to represent electron spins inside each orbital box. In the orbital notation of scandium, the single 3d electron is shown unpaired, following Hund’s rule.
Why Is the Orbital Diagram of Scandium Important?
The scandium orbital diagram helps explain oxidation states, magnetic properties, and periodic trends. It is especially useful for students learning atomic structure through diagrams.
Frequently Asked Faqs
Here are Answer to common question about making Scandium Orbital Diagram.
Q1: What does the orbital diagram for scandium show?
It shows how 21 electrons are arranged in scandium’s atomic orbitals.
Q2: How many unpaired electrons are in the scandium orbital diagram?
There is one unpaired electron in the 3d orbital.
Q3: Why does scandium have a d orbital in its diagram?
Because scandium is a transition element with electrons entering the 3d subshell.
Q4: Is scandium orbital notation different from electron configuration?
Yes, orbital notation visually shows electron spins and pairing.
Q5: Where are the valence electrons in the sc orbital diagram?
They are found in the 4s and 3d orbitals.






