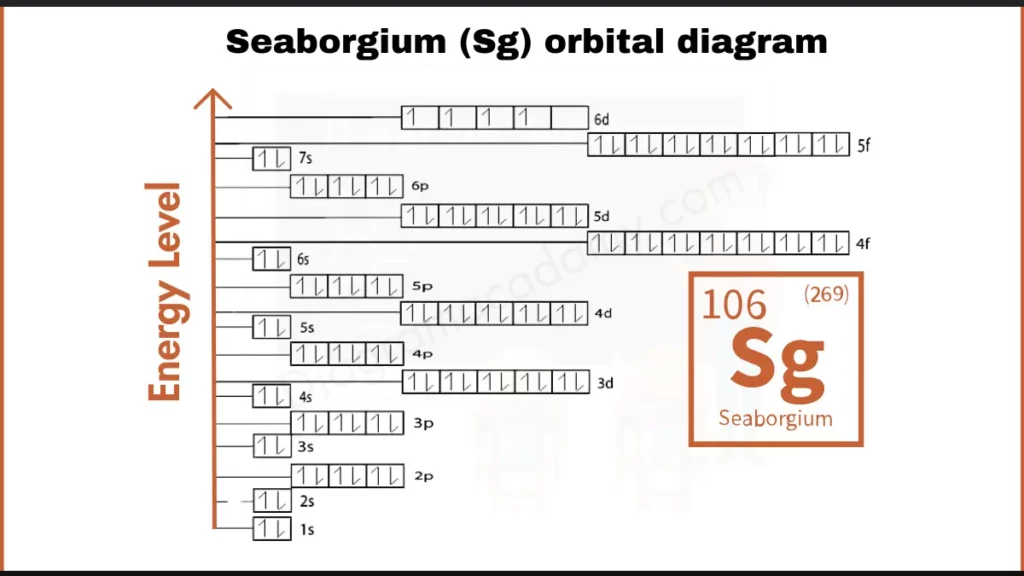
How to write Orbital Diagram for Seaborgium?
Seaborgium (Sg), with 106 electrons, has a complex configuration written as [Rn] 5f¹⁴ 6d⁴ 7s². Here, [Rn] represents the filled electron shells of Radon. The valence electrons lies in 6d subshell, which holds just four electrons (6d⁴). This incomplete configuration is believed to be a significant factor in Seaborgium’s unique bonding behavior.