What Does the Orbital Diagram for Selenium Illustrate?
The orbital diagram for selenium visually shows how electrons are distributed among atomic orbitals in a selenium atom. Instead of using only numerical electron configurations, the selenium orbital diagram uses boxes and arrows to represent orbitals and electron spins, making the atomic structure easier to understand through diagrams.
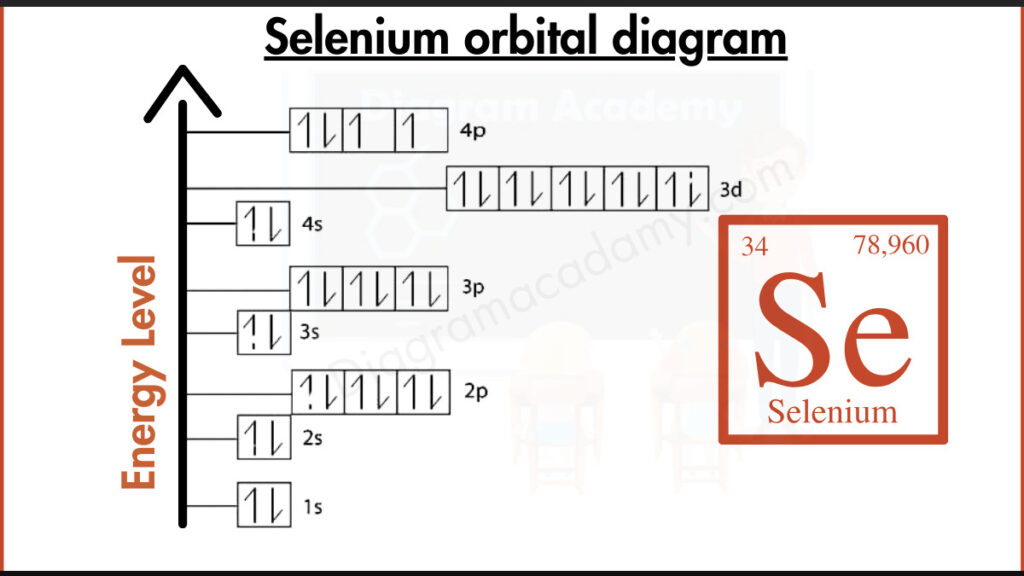
Which Atomic Details Are Needed to Understand Selenium Orbitals?
Selenium has the chemical symbol Se and an atomic number of 34, meaning it contains 34 electrons. It is located in period 4 and belongs to the p-block of the periodic table. These facts directly influence the structure of the orbital diagram of selenium, especially the filling of p orbitals in the outer shell.
What Is the Electron Configuration Behind the Selenium Orbital Diagram?
The electron configuration of selenium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁴
This configuration is converted into the orbital diagram for Se by assigning each orbital a box and placing electrons according to standard filling rules.
How Are Electrons Placed in the Orbital Diagram of Selenium?
In the orbital diagram selenium, electrons fill orbitals from lower to higher energy levels following the Aufbau principle. The 4s orbital fills before the 3d and 4p orbitals. In the 4p subshell, electrons occupy separate orbitals before pairing, which results in two unpaired electrons visible in the diagram.
How Can the Selenium Orbital Diagram Be Read Visually?
The orbital diagram of selenium is typically arranged vertically to show increasing energy. Lower-energy orbitals appear at the bottom, while higher-energy orbitals such as 4p appear near the top. This layout helps readers quickly identify valence electrons and orbital occupancy.
What Does Selenium Orbital Notation Represent?
Selenium orbital notation uses arrows to represent electron spin within each orbital box. Paired arrows indicate paired electrons, while single arrows show unpaired electrons. This notation explains selenium’s bonding behavior and reactivity.
Why Is the Orbital Diagram for Se Important in Chemistry?
The orbital diagram for selenium helps explain valence electrons, oxidation states, and chemical bonding. It is widely used in education, exams, and diagram-based learning platforms to simplify atomic structure concepts.
Frequently Asked FAQs
Here are Answer to Common question about Orbital Diagram for Selenium (Se).
Q1: What does the orbital diagram for selenium show?
It shows how 34 electrons are arranged in selenium’s atomic orbitals.
Q2: How many valence electrons appear in the selenium orbital diagram?
Selenium has six valence electrons shown in the 4s and 4p orbitals.
Q3: Why are there unpaired electrons in the orbital diagram of selenium?
Because electrons fill separate p orbitals before pairing, following Hund’s rule.
Q4: Is orbital notation for selenium different from electron configuration?
Yes, orbital notation visually shows electron spin and pairing, while configuration lists electron numbers.
Q5: Why is the orbital diagram for Se useful for students?
It helps students understand bonding, reactivity, and periodic trends through clear visuals.






