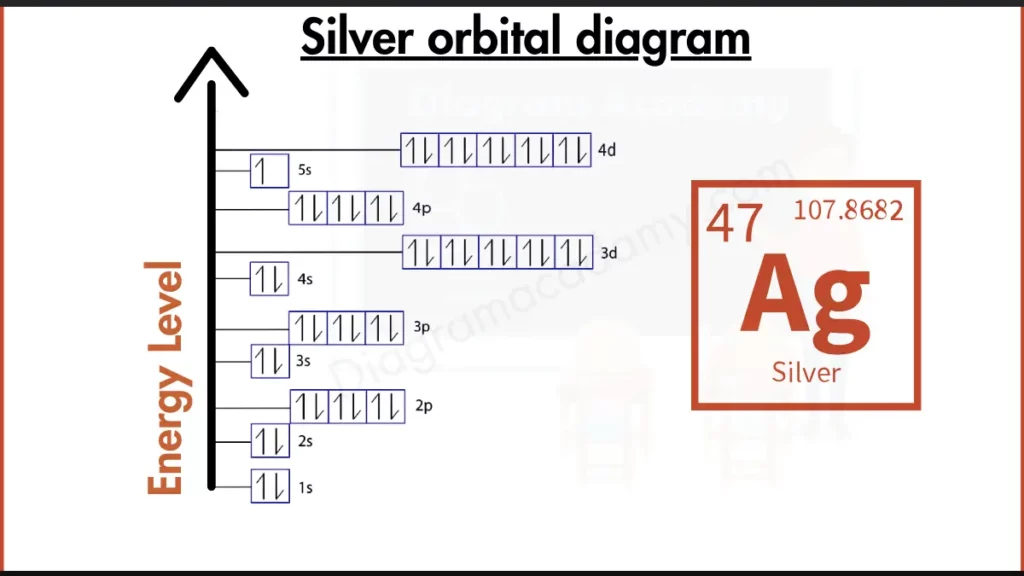
How to write the Orbital Diagram For Silver?
Silver (Ag) shines bright with a stable outer shell, similar to the noble gases. Its electron configuration, written as [Kr] 4d¹⁰ 5s¹, showcases a peculiarity. Even though transition metals like Silver typically have partially filled d-orbitals, Silver gains extra stability by completely filling its 4d subshell before placing an electron in the 5s subshell. This unique arrangement, with a full 4d subshell and a single electron in the 5s, makes Silver more stable than most transition metals and contributes to its low reactivity, resembling the behavior of the noble gases with their full outer shells.