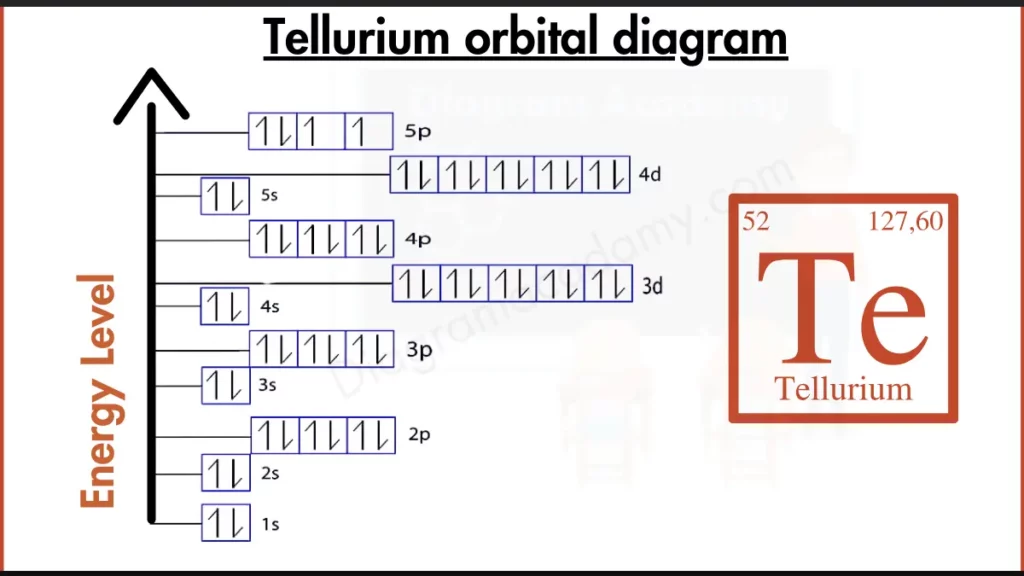
How to Write the Orbital Diagram for Tellurium?
outer shell of Tellurium (Te) holds six electrons, written as [Kr] 4d¹⁰ 5s² 5p⁴. This configuration shows Tellurium has some ways to go for ultimate stability. While the 4d subshell is filled and the 5s subshell holds two electrons, the 5p subshell isn’t completely full. This incomplete outer shell, compared to noble gases with packed outer shells, makes Tellurium more reactive. Tellurium seeks to gain two more electrons to fill its 5p subshell and achieve the stability enjoyed by its noble gas neighbors.