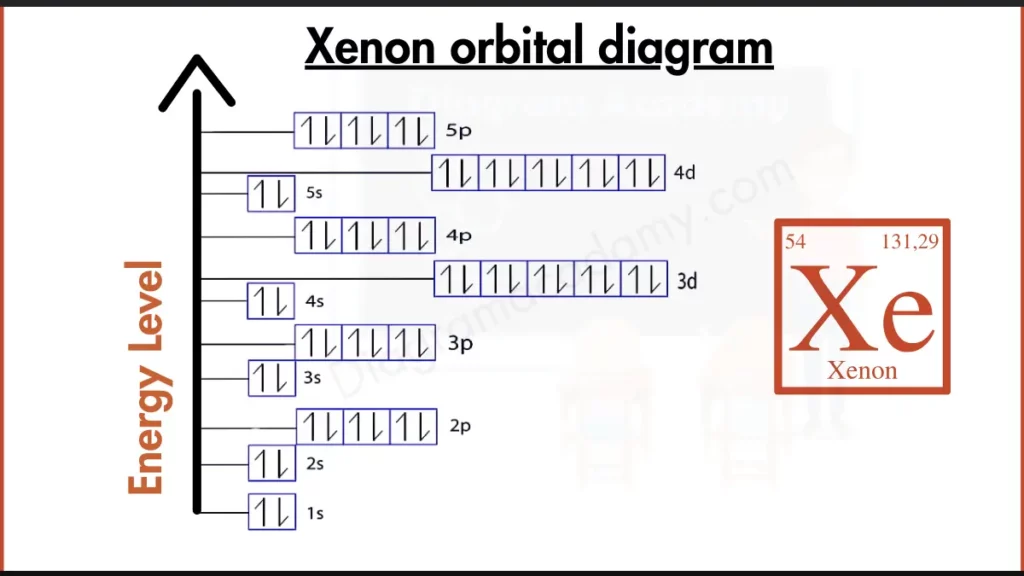
How to Write the Orbital Diagram for Xenon?
Xenon (Xe) throws a perfect strike in the game of noble gas stability. Its electron configuration, [Kr] 4d¹⁰ 5s² 5p⁶, boasts a completely filled outer shell. This configuration, with electrons occupying all six spots in the 5p subshell, mirrors other noble gases like Krypton (Kr). This complete outer shell is the key to Xenon’s unreactive nature. Just like other members of the noble gas club, Xenon has no need to gain or lose electrons to achieve a stable state, making it very stable and resistant to forming bonds.