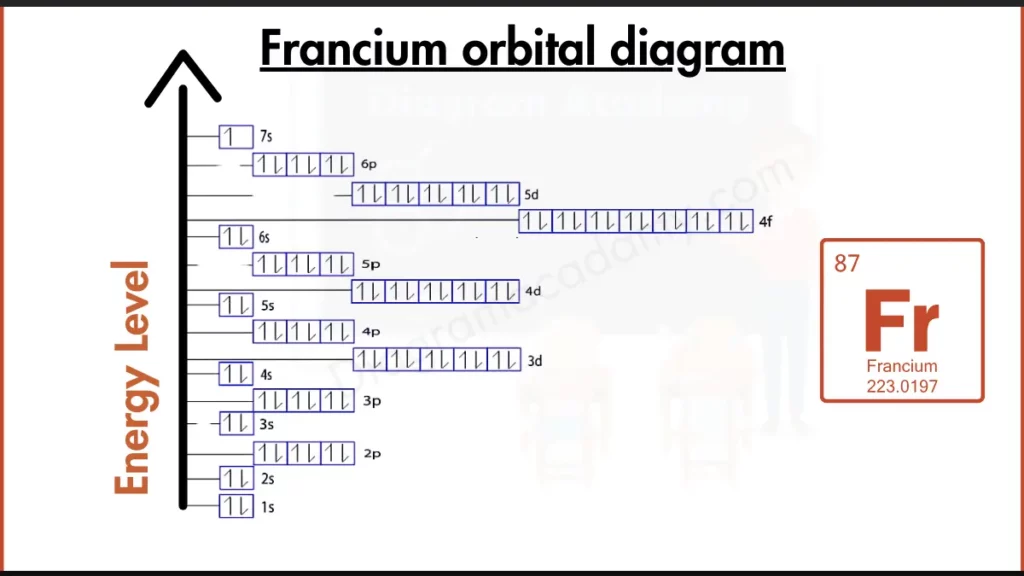
How to Write the Orbital Diagram for Francium?
Francium (Fr) is the lone wolf. It has just one electron in its outermost shell, following the configuration [Rn] 7s¹. This single electron makes it highly unstable, unlike the stable noble gases with full outer shells. This single electron also hints at Francium’s high reactivity.