What Is the Orbital Diagram for Rubidium?
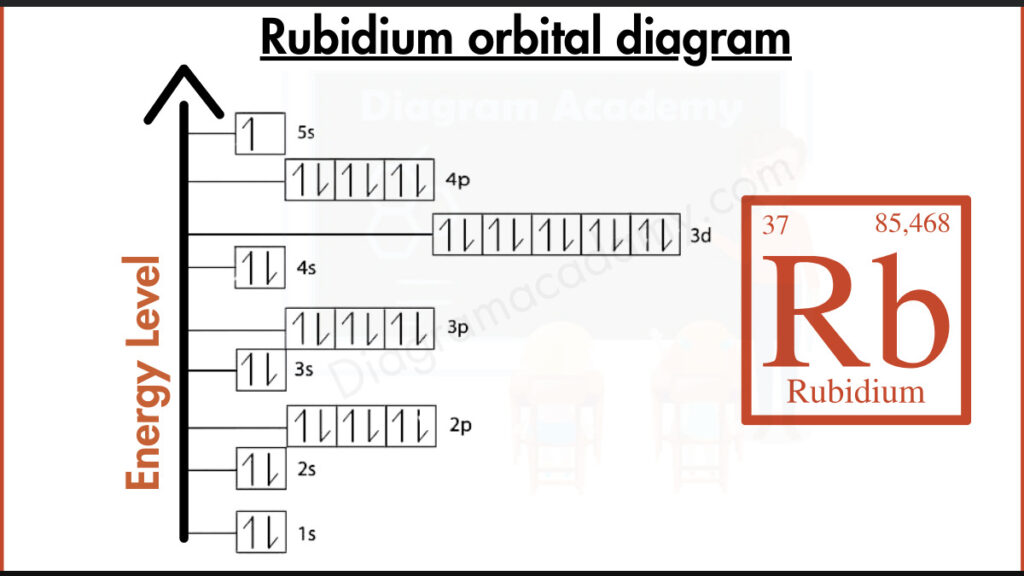
The orbital diagram for rubidium visually shows how electrons are distributed in rubidium’s atomic orbitals. The rubidium orbital diagram uses arrows and boxes to clearly display electron arrangement, making it ideal for image-based learning.
What Atomic Information Is Needed for the Rubidium Orbital Diagram?
Rubidium has the symbol Rb and an atomic number of 37, meaning it has 37 electrons. It is a period-5 alkali metal, which strongly influences the rb orbital diagram.
How Does Electron Configuration Define the Orbital Diagram of Rubidium?
The electron configuration of rubidium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s¹
This configuration forms the basis of the orbital diagram of rubidium, showing one valence electron.
How Is the Orbital Filling Diagram for Rubidium Constructed?
In the orbital filling diagram for rubidium, electrons fill lower-energy orbitals first. The single electron in the 5s orbital appears unpaired, which is clearly shown in the orbital diagram for Rb.
How Are Energy Levels Shown in the Orbital Diagram of Rb?
The orbital diagram of Rb places the 5s orbital at the highest energy level. This vertical structure helps users quickly identify rubidium’s outermost electron.
What Is Orbital Notation for Rubidium?
Orbital notation for rubidium shows a single arrow in the 5s orbital, representing its lone valence electron. This explains rubidium’s high reactivity.
Why Is the Orbital Diagram for Rubidium Important?
The orbital diagram for rubidium explains alkali metal behavior, reactivity, and bonding, making it essential for chemistry diagrams and study resources.
Frequently Asked FAQs
Here are the answer to common question about Orbital Diagram for Rubidium.
Q1: What does the orbital diagram for rubidium show?
It shows 37 electrons with one unpaired electron in the 5s orbital.
Q2: Why is rubidium highly reactive according to its orbital diagram?
Because it has one loosely held valence electron.
Q3: Where is the highest energy electron in the orbital diagram of Rb?
In the 5s orbital at the top of the diagram.






