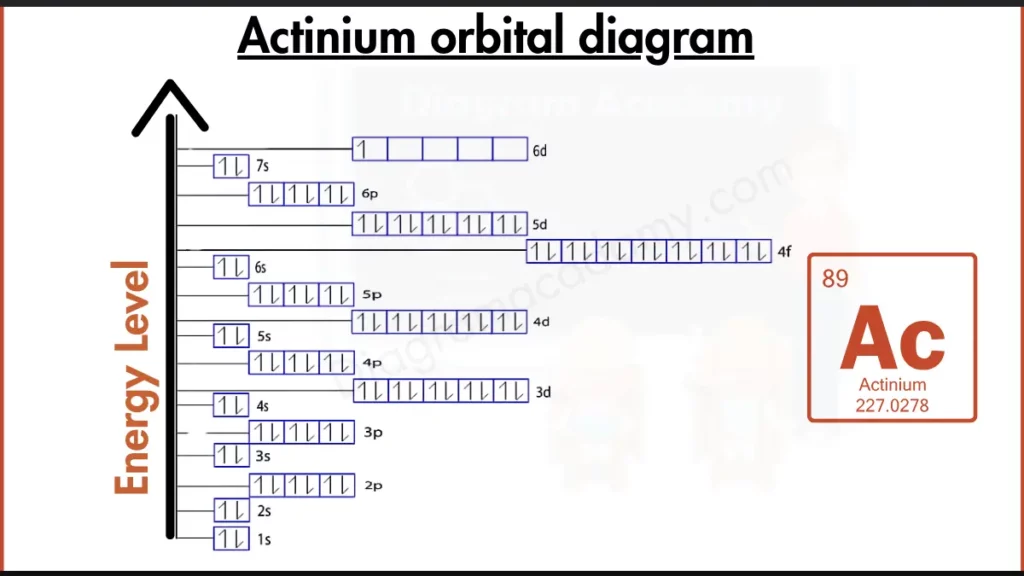
How to write the Orbital Diagram for Actinium?
Actinium (Ac) stands out from the others. Unlike its heavier neighbors, Actinium has a mostly filled outer shell, with electrons in the 6d and 7s subshells ([Rn] 6d¹ 7s²). This configuration, closer to noble gases, hints at some stability, but the presence of an electron in the 6d subshell suggests Actinium might be slightly more reactive than a true noble gas