What Is the Arsenic Orbital Diagram?
The arsenic orbital diagram is a visual representation that shows how electrons are distributed in the atomic orbitals of an arsenic atom. Unlike a simple configuration, the orbital diagram for arsenic uses boxes and arrows to illustrate individual orbitals and electron spins, making electron arrangement easier to understand through diagrams.
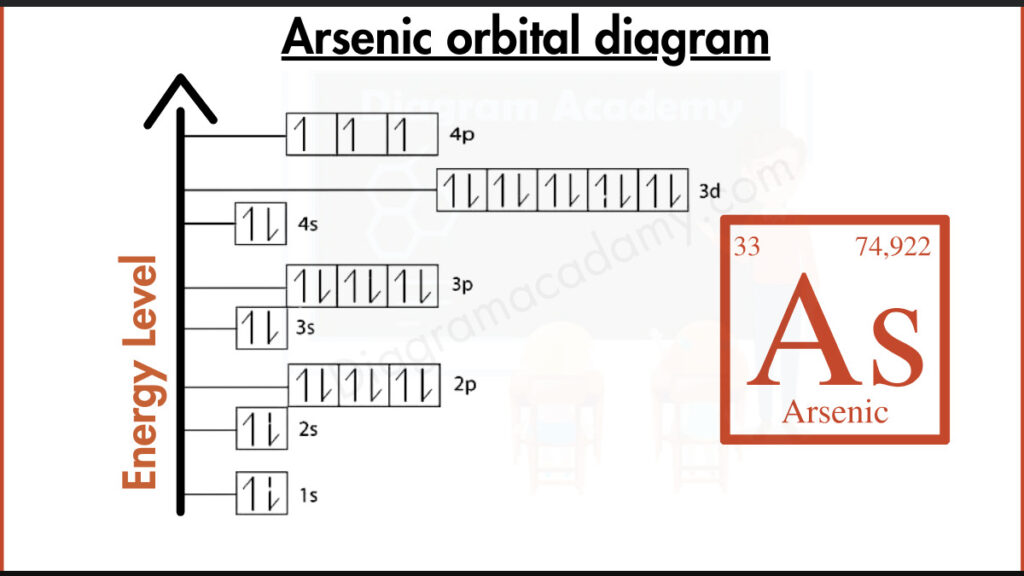
What Atomic Details Are Needed for the Orbital Diagram for Arsenic?
To correctly understand the orbital diagram of arsenic, basic atomic information is required. Arsenic has the symbol As and an atomic number of 33, meaning it contains 33 electrons. It is a period-4, p-block element, which explains the presence of p orbitals in the orbital diagram arsenic.
How Does Electron Configuration Shape the Arsenic Orbital Diagram?
The electron configuration of arsenic is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³
This configuration forms the basis of the arsenic orbital notation and determines how electrons are placed into each orbital in the diagram.
How Is the Orbital Diagram of Arsenic Filled Step by Step?
In the orbital diagram of As, electrons fill orbitals according to the Aufbau principle, starting from lower energy levels. The 4s orbital fills before the 3d and 4p orbitals. In the 4p subshell, electrons occupy separate orbitals before pairing, which is clearly shown in the orbital diagram for As.
How Are Energy Levels Shown in the Orbital Diagram of As?
The orbital diagram of arsenic is arranged vertically to represent increasing energy. Lower orbitals such as 1s and 2s appear at the bottom, while higher-energy orbitals like 4p appear near the top. This layout helps learners quickly interpret the diagram of As.
What Is Orbital Notation for Arsenic?
Orbital notation for arsenic uses arrows to show electron spin within each orbital. In arsenic, the three electrons in the 4p orbitals remain unpaired, which is clearly visible in arsenic orbital notation and explains its chemical behavior.
Why Is the Orbital Diagram for As Important to Study?
The orbital diagram for arsenic helps explain bonding, reactivity, and periodic trends. It is commonly used in chemistry education, exams, and visual learning resources where diagrams simplify complex concepts.
Frequently Asked FAQs
Here are the answer to common question about Arsenic Orbital Diagram (As).
Q1: What does the arsenic orbital diagram show?
The arsenic orbital diagram shows how 33 electrons are arranged in atomic orbitals using arrows and boxes.
Q2: How many unpaired electrons are in the orbital diagram of arsenic?
The orbital diagram of arsenic shows three unpaired electrons in the 4p orbitals.
Q3: Why does the orbital diagram for arsenic include p orbitals?
Arsenic is a p-block element, so its outer electrons occupy p orbitals.
Q4: Is orbital notation for arsenic different from electron configuration?
Yes, orbital notation for arsenic visually shows electron spin and pairing, while configuration only lists electron counts.
Q5: Where are the highest energy electrons shown in the orbital diagram of As?
In the orbital diagram of As, the highest energy electrons appear in the 4p orbitals at the top of the diagram.






