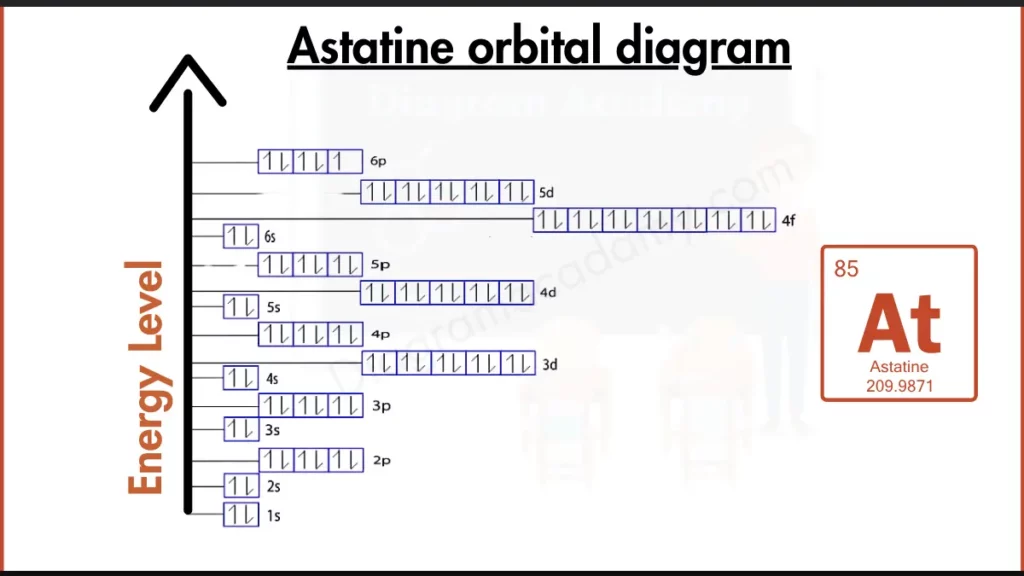
How to Write the Orbital Diagram for Astatine?
Astatine (At) joins the party with the halogens. Its outer shell isn’t quite full, holding five electrons in the 6p subshell ([Xe] 4f¹⁴ 5d¹⁰ 6s² 6p⁵). This incomplete configuration, similar to other halogens like Chlorine (Cl), makes Astatine very reactive. Unlike the noble gases with packed outer shells, Astatine seeks to form bonds to achieve stability.