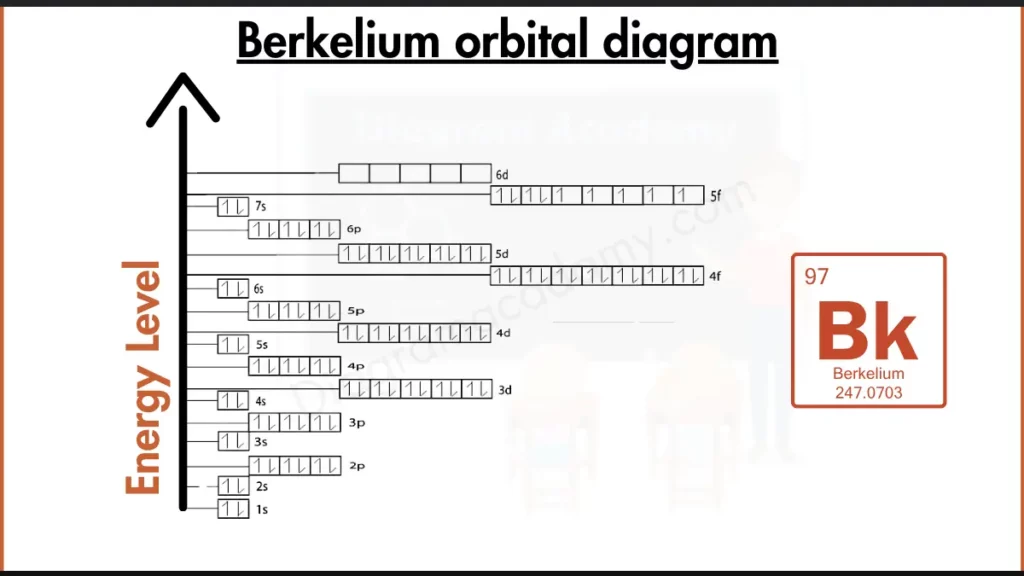
How to write the Orbital Diagram for Berkelium?
Berkelium (Bk) isn’t like the stable noble gases. Its outer shell isn’t full, instead having electrons in the 5f and 7s subshells. This incompleteness is reflected by its electron configuration, [Rn] 5f⁹ 7s², and makes Berkelium more reactive. The bracketed [Rn] represents filled inner shells, similar to Radon (Rn).