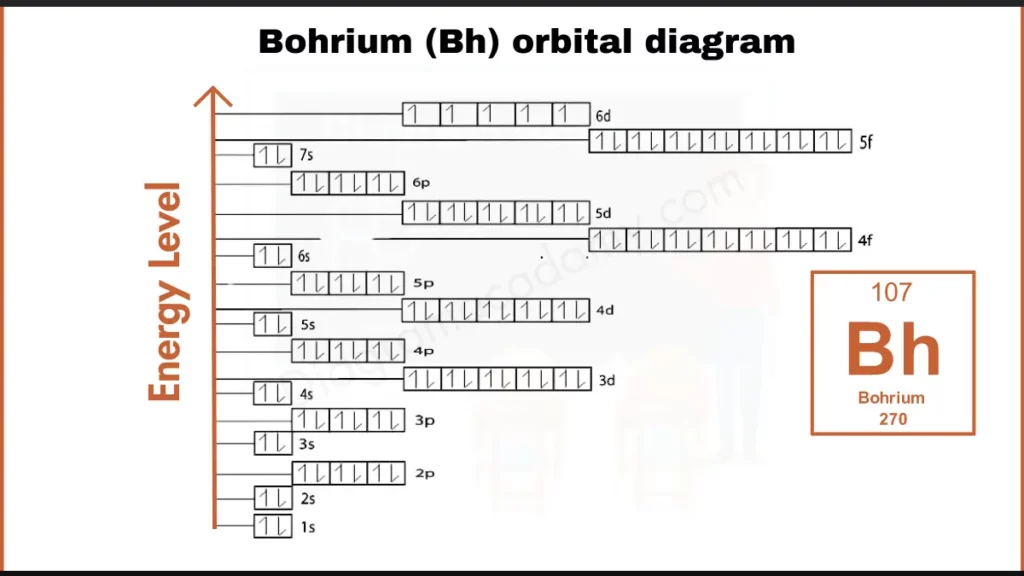
How to Write the Orbital Diagram for Bohrium?
The element Bohrium (Bh) possesses a complex electron configuration with 107 electrons. This can be represented as [Rn] 5f¹⁴ 6d⁵ 7s², where [Rn] denotes the filled electron shells of Radon. The outermost 6d subshell holds only five electrons, contributing to Bohrium’s unique bonding behavior.