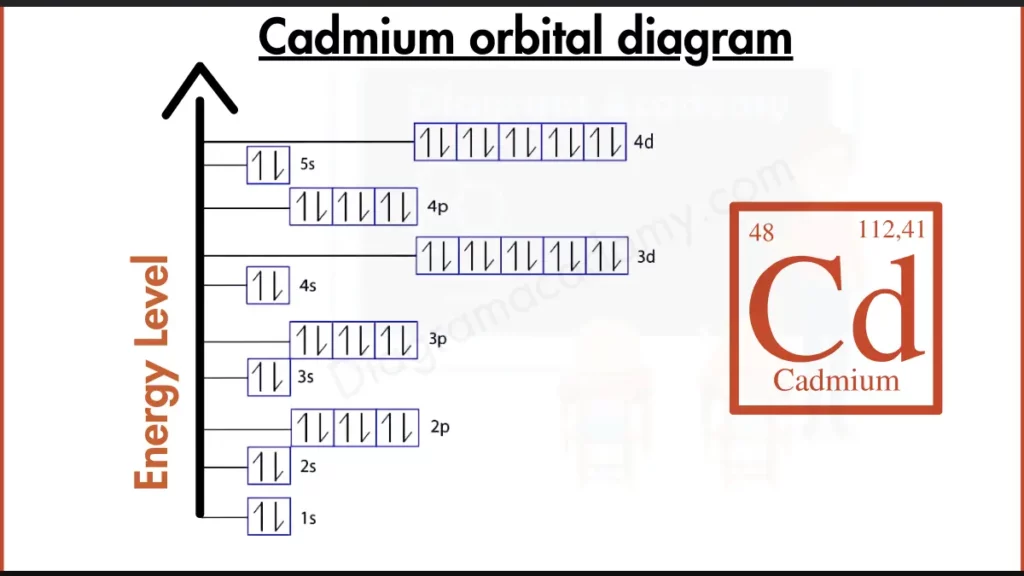
How to Write the Orbital Diagram for Cadmium?
Cadmium (Cd) isn’t as stable as the noble gases, but it’s not far off. Its outer shell holds two electrons in the 5s subshell, written as [Kr] 4d¹⁰ 5s². This configuration, with a filled 4d subshell and electrons in the 5s, makes Cadmium somewhat stable but still reactive compared to true noble gases. It hasn’t quite achieved the full outer shell magic for ultimate stability.