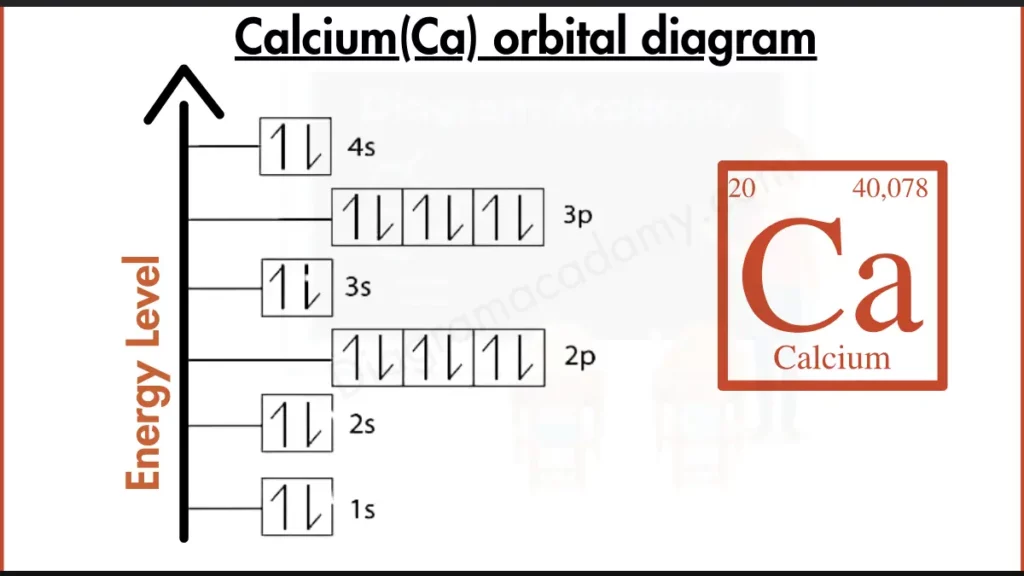
How to write the Orbital Diagram for Calcium?
Calcium (Ca) has 20 electrons filling its shells. A common way to describe this is [2, 8, 8, 2]. This means the electrons fill up the shells closest to the nucleus first. The first shell holds 2 electrons, the second and third shells each hold 8 electrons, and the outermost shell holds the remaining 2 electrons.