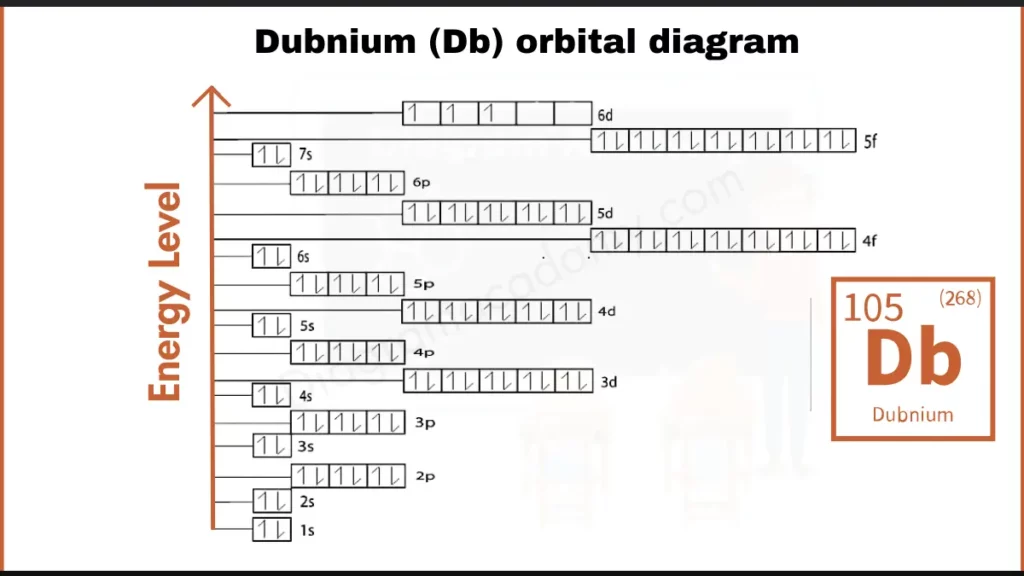
How to write Orbital Diagram for Dubnium?
Dubnium (Db) has 105 electrons. Its configuration, [Rn] 5f¹⁴ 6d³ 7s² with [Rn] representing filled Radon shells, reveals an incomplete outermost shell. The 6d subshell holds just three electrons (6d³), contributing to Dubnium’s unique bonding behavior.