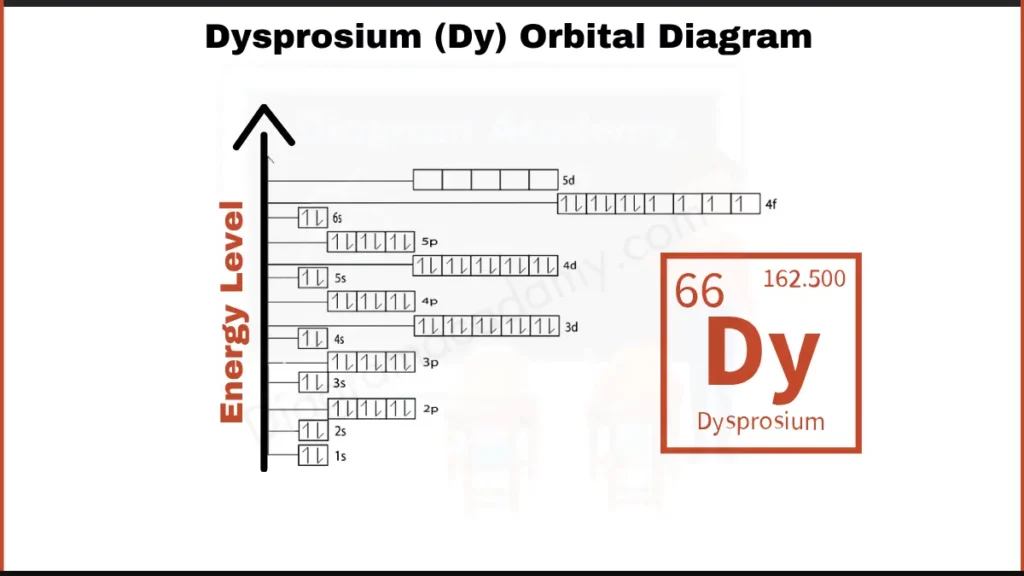
How to Write the Orbital Diagram for Dysprosium?
Dysprosium (Dy) has 66 electrons orbiting its nucleus. These electrons fill shells in a specific order. Imagine these shells as layers around the nucleus. A simpler way to describe the arrangement is by focusing on the outer electrons. Dysprosium, similar to other lanthanides, has 10 electrons in its 4f subshell and 2 electrons in the outermost shell, denoted by 6s.