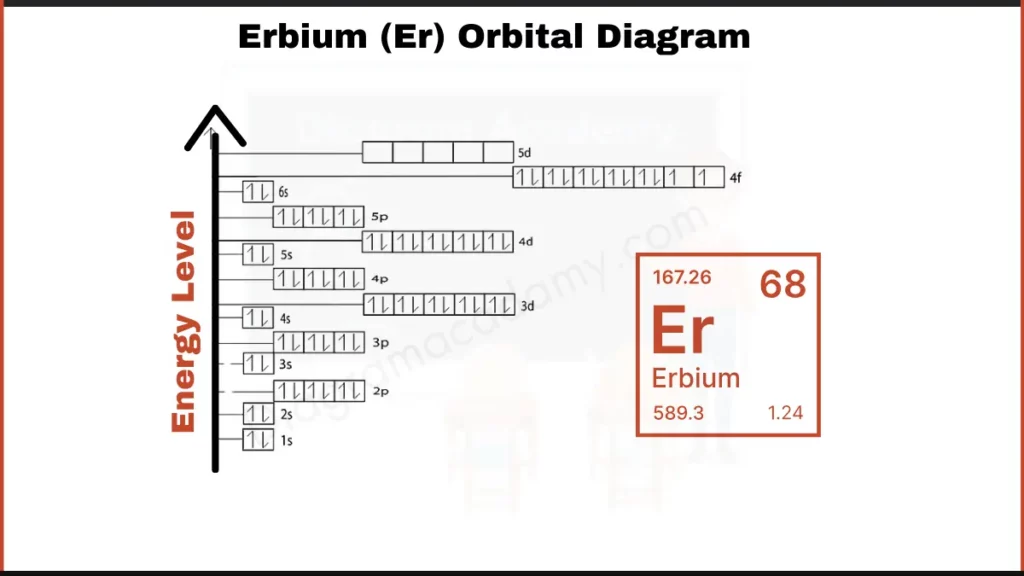
How to Write the Orbital Diagram for Erbium?
Erbium (Er), like dysprosium, has a total of 68 electrons orbiting its nucleus. These electrons fill shells in a specific order. We can focus on the outer electrons for a simpler picture. Erbium, along with other lanthanides, has its outer electrons arranged with 12 electrons in the 4f subshell and 2 in the outermost 6s subshell.