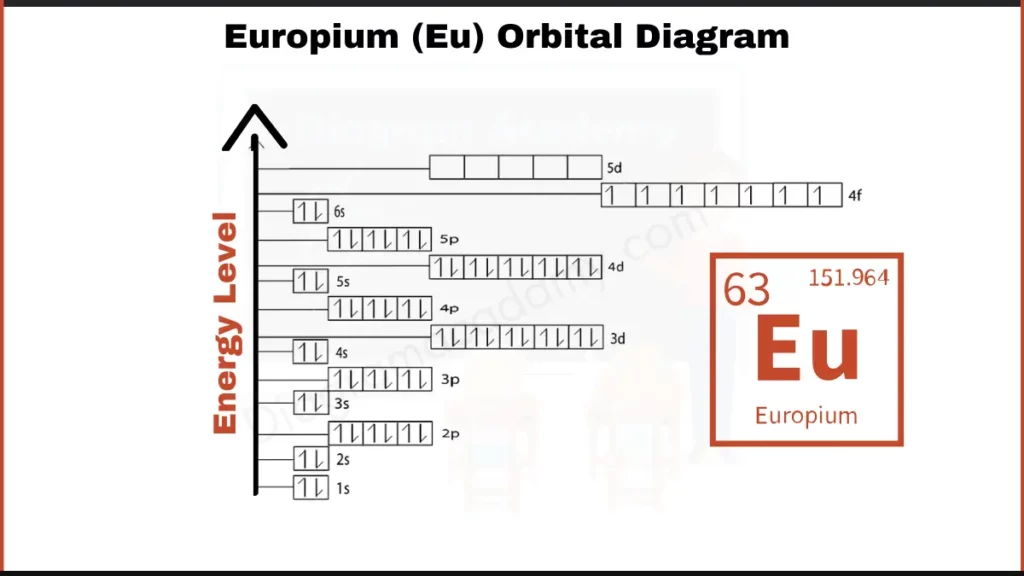
How to Write the Orbital Diagram for Europium?
Europium (Eu) has 63 electrons orbiting its nucleus. These electrons fill shells in a specific order. Like layers around the core, these shells hold a certain number of electrons. Europium, similar to other lanthanides, has a unique arrangement with 7 electrons in its 4f subshell and 2 electrons in the outermost shell (6s). This configuration influences its chemical properties.