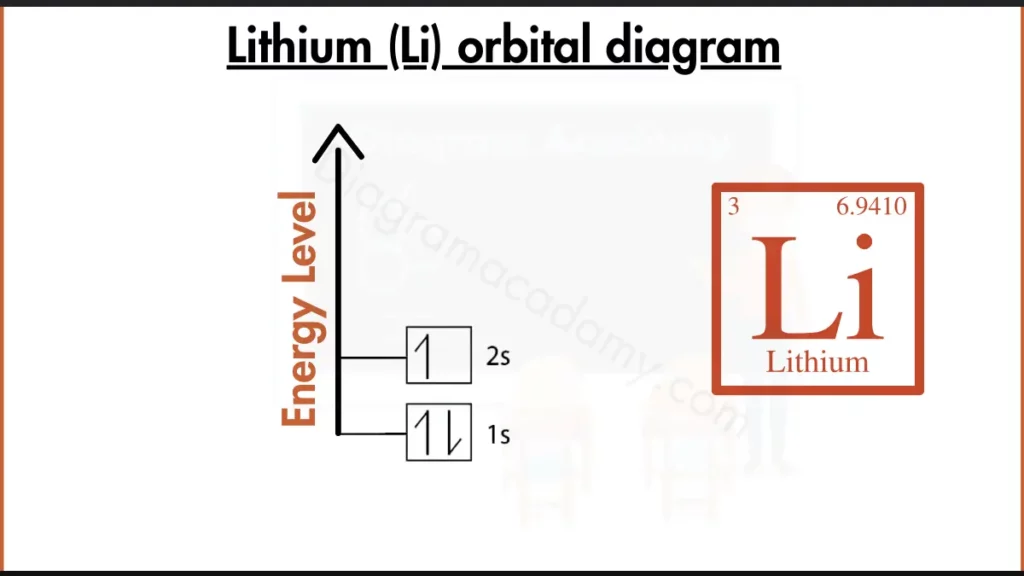
How to Write the Orbital Diagram for Lithium?
Lithium (Li) is element with 3 electrons, has the configuration 1s²2s¹. This fills the inner subshell (1s) and places the remaining electron in the second energy level’s s subshell (2s¹). This single valence electron in lithium makes it reactive, readily losing the electron to achieve a stable, full inner shell configuration like helium.