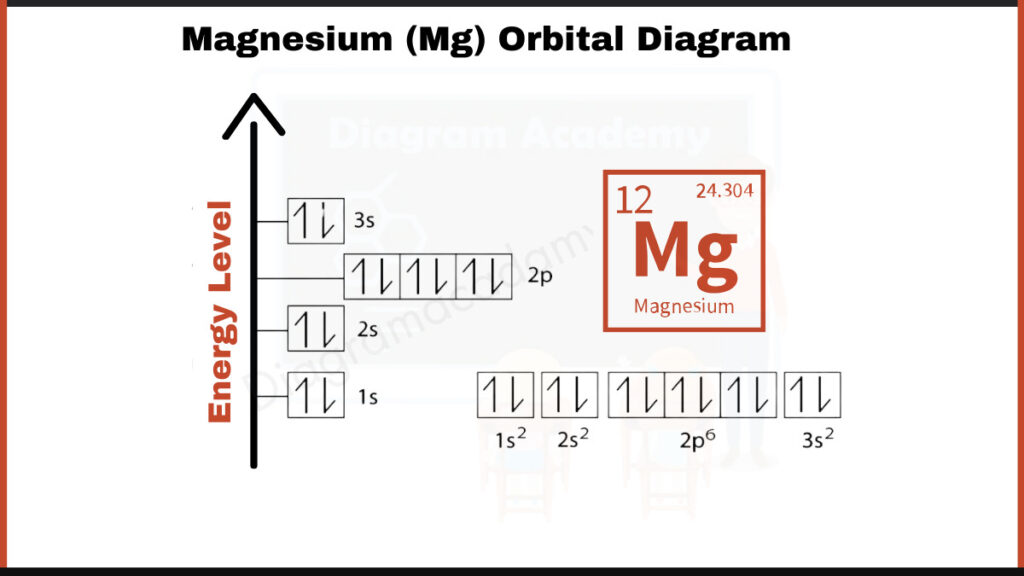
How to write the Orbital Diagram for Magnesium?
Magnesium (Mg) has 12 electrons. They fill shells in a specific order: [2, 8, 2]. The first number, 2, represents the two electrons in the innermost shell closest to the nucleus. The second number, 8, corresponds to the eight electrons in the next shell. The final 2 electrons occupy the outermost shell of magnesium.