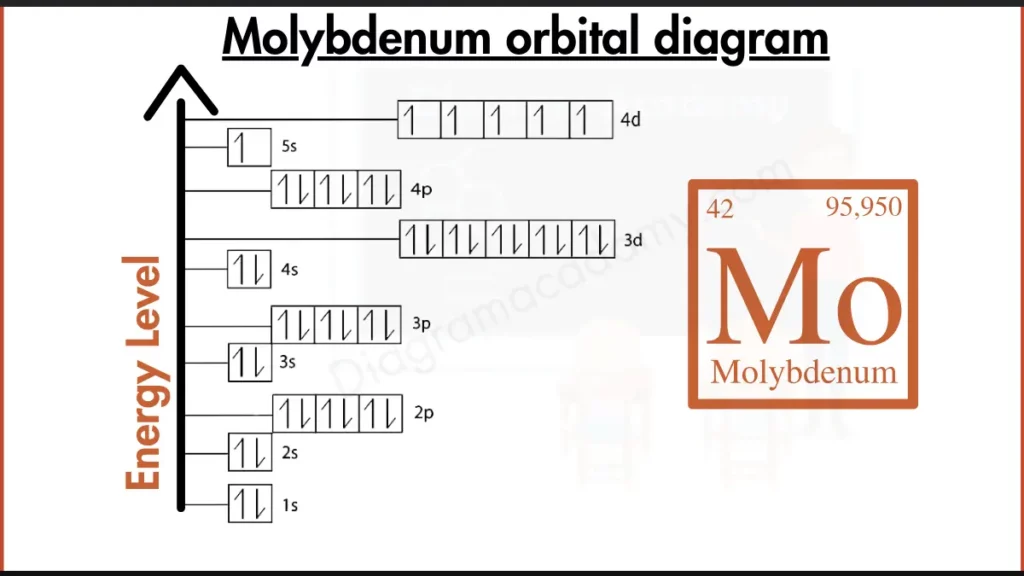
How to write the Orbital Diagram for Molybdenum?
Molybdenum (Mo) joins the club of reactive metals. Its outer shell isn’t full, containing electrons in both the 4d and 5s subshells ([Kr] 4d⁵ 5s¹). This incomplete configuration, similar to Technetium (Tc), makes Molybdenum more reactive compared to the stable, full outer shells of noble gases. It seeks to reach a more stable state by gaining or losing electrons to achieve a filled outer shell.