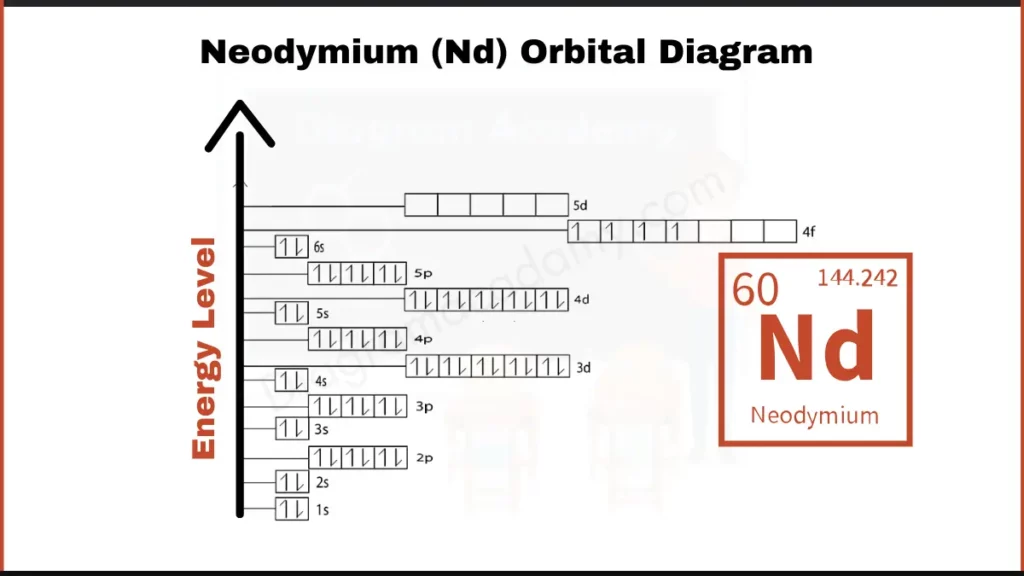
How to Write the Orbital Diagram for Neodymium?
Neodymium (Nd) has 60 electrons arranged in shells around its nucleus. Similar to other lanthanides, the configuration follows a pattern, with 4 electrons in the 4f subshell and 2 in the outermost 6s subshell. This arrangement of electrons plays a role in determining Neodymium’s chemical properties.