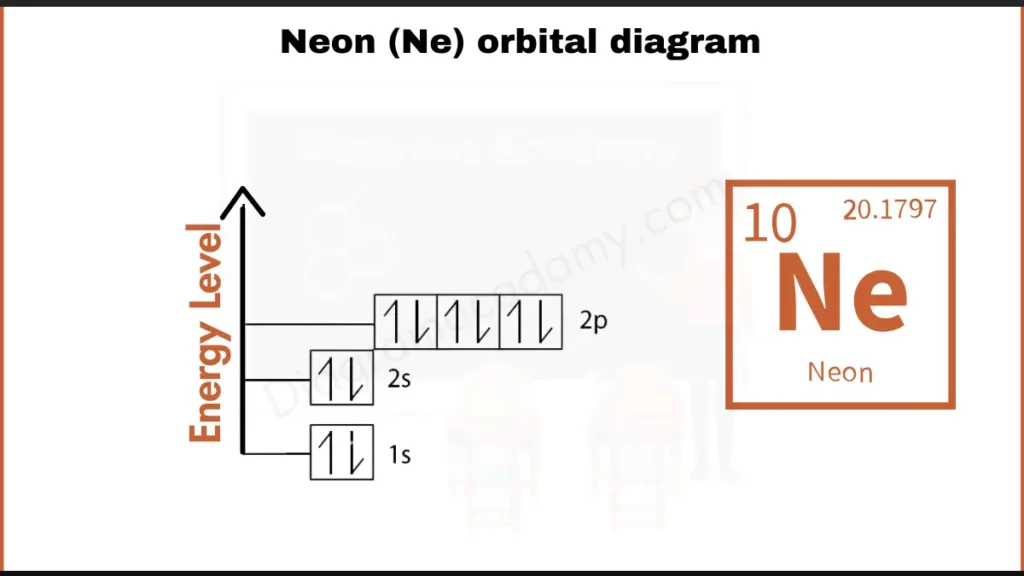
How to Write the Orbital Diagram For Neon?
Neon (Ne), with 10 electrons, has the configuration 1s²2s²2p⁶. This fills all orbitals in its second energy level (2s and 2p). It makes neon a stable noble gas. The orbital diagram reflects this with two electrons in each of the 1s, 2s, and all three 2p orbitals (up to six electrons).