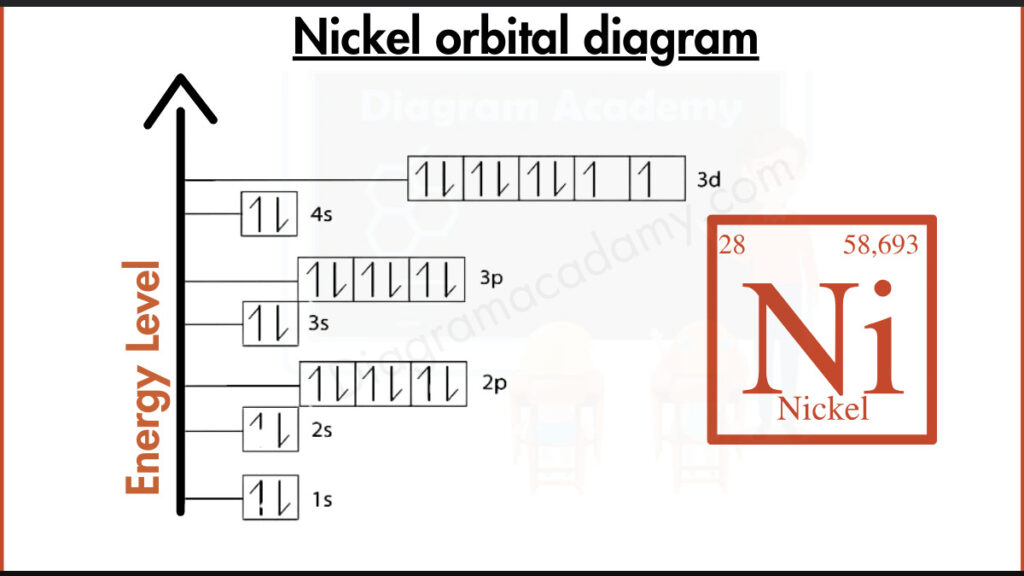
How to write the Orbital Diagram for Nickel?
Nickel (Ni) keeps 28 electrons in its atomic neighborhood. The inner two shells hold a total of 10 electrons. Next comes a filled inner shell, tucking away 10 more. Finally, the outermost shell holds the important ones: 8 valence electrons. These are the players on the frontline, determining how nickel interacts with other elements through chemical bonds.