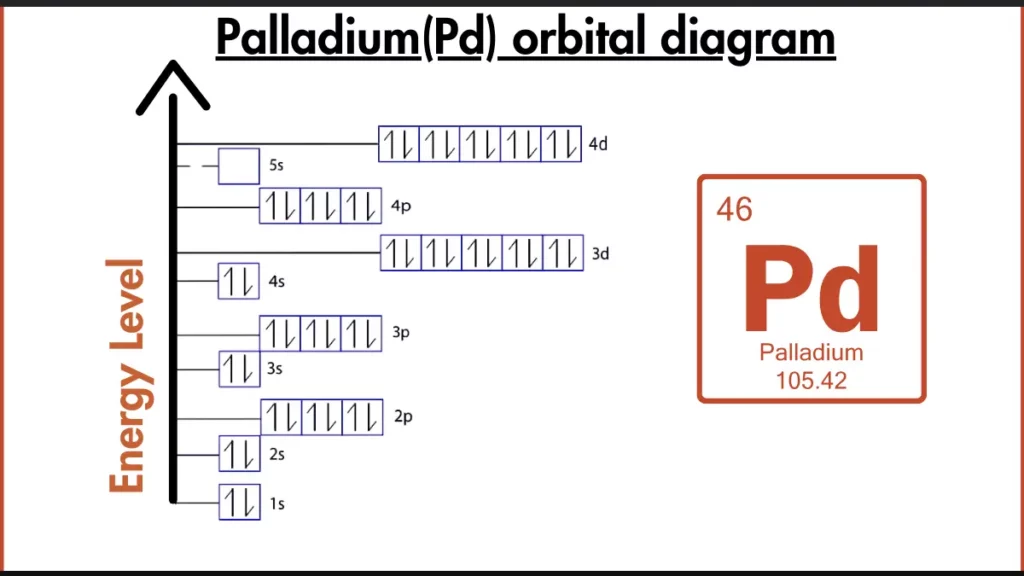
How to Write the Orbital Diagram for Palladium?
While most transition metals have partially filled d-orbitals, Palladium goes for stability. Its electron configuration, [Kr] 4d¹⁰, shows a completely filled 4d subshell. This isn’t typical, but it makes Palladium more stable for it. So, even though it’s not like the noble gases with full outer shells, Palladium prioritizes stability with its electron arrangement