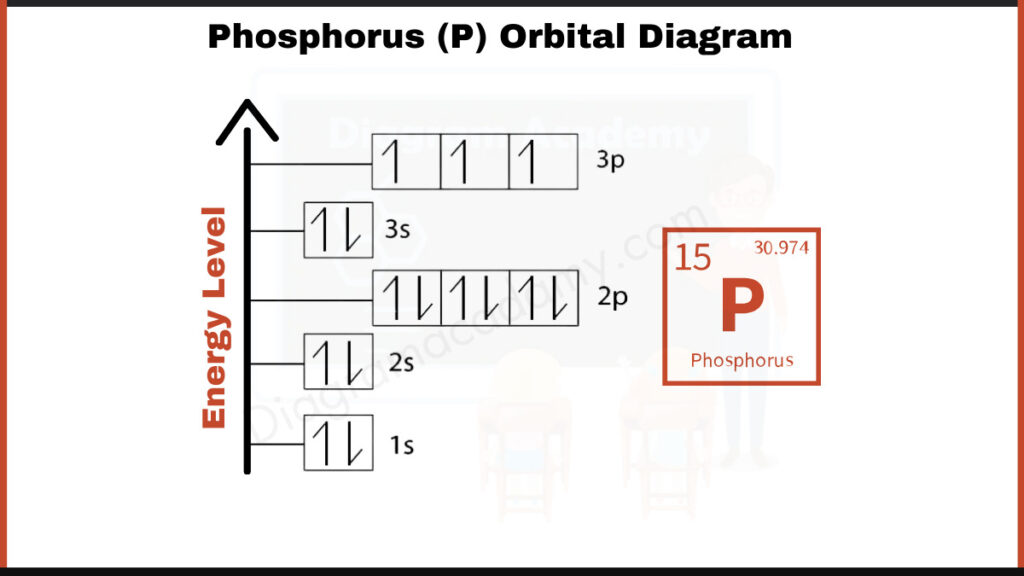
How to write the Orbital Diagram for Phosphorus?
Phosphorus (P) has 15 electrons. They fill shells in a specific order: [2, 8, 5]. This means the first shell holds 2 electrons, the second shell holds 8 electrons, and the third shell has the remaining 5 electrons. This shorthand notation is a way to efficiently describe the electron arrangement in Phosphorus.