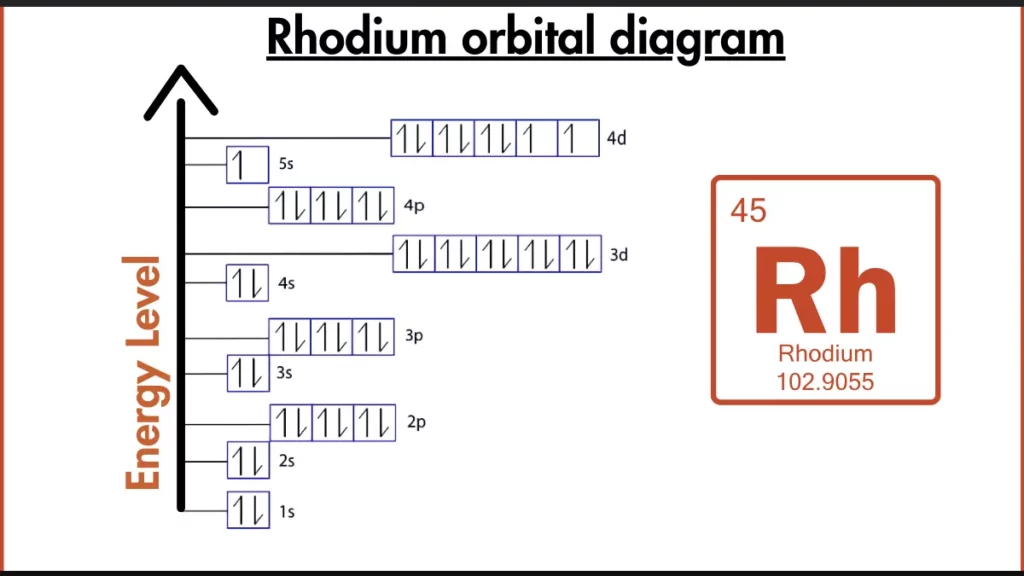
How to write the Orbital Diagram of Rhodium?
Rhodium (Rh) isn’t looking to play by the usual rules. While most transition metals have partially filled d-orbitals, Rhodium fills its 4d subshell before the 5s. Its electron configuration, [Kr] 4d⁸5s¹, reflects this unusual stability. This arrangement, with a full 4d subshell and one electron in the 5s, makes Rhodium more stable than expected for a transition metal, but it still isn’t quite as unreactive as the noble gases with their completely filled outer shells.